Hyphal
tropismsA
tropism is an orientation response of a hypha to an
external stimulus. An example is shown in Figure D, where
encysted zoospores of Pythium aphanidermatum (Oomycota)
were allowed to germinate in water, with a nutrient-rich
agar block positioned on one side of the cyst clusters.
Zoospore cysts of the oomycota have a pre-determined
point of germination, so the young hyphae emerged in all
directions but then they reorientated and grew towards
the nutrient-rich agar (to the right in the images
below).
This type of tropism to
organic nutrients is found in several members of the
fungus-like group oomycota, including the "water moulds"
such as Saprolegnia and Achlya species,
some of which parasitise freshwater fish. However,
tropism to organic nutrients does not seem to occur in
the true (chitin-walled) fungi. Instead, these have other
forms of tropism, discussed below.
|
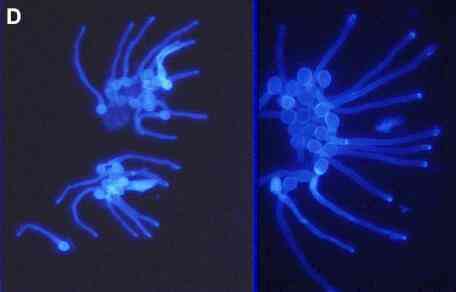
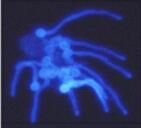
Figure
D.
Young hyphae (germ
tubes) growing from zoospore cysts of Pythium
aphanidermatum, seen at different magnifications in
the two images. The cysts germinated from a predetermined
(fixed) point but the hyphae then grew towards a
nutrient-rich agar block (malt extract and peptone) to
the right-hand side (not shown). The cells were stained
with the fluorescent brightener, Calcofluor, and
photographed with a fluorescence microscope, using
near-UV illumination.
|
Spore
tropismsUnlike the
zoospore cysts mentioned above, the spores of most fungi
do not have a fixed point of germination. Instead they
can germinate from almost any point, which can be
influenced by external factors. A spectacular example of
this is shown in Figures E-G, where spores of Idriella
bolleyi (deuteromycota) were sprayed onto the roots
of young, aseptic wheat seedlings growing on a thin film
of water agar.
The spores on the surface of living
root hairs were almost always seen to germinate away from
the root hair, and the germ tubes continued to grow away
(see the two spores labelled "s" in Fig. E). In
contrast, spores on the surface of dead root hairs
germinated towards them, and the germ tubes coiled round
the root hairs (Figure F) and penetrated them (Fig. G,
arrowhead).
|
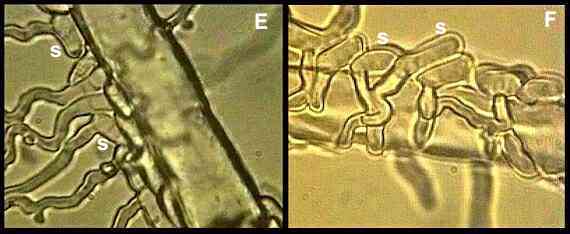
Figures
E, F.
Spores (s) of Idriella
bolleyi germinating away from living cereal root
hairs (E) but towards dead root hairs (F). Images taken
from videotapes (Allan et al., 1992).
|
The factors that cause the differential tropism of I.
bolleyi to living and dead root cells are still
unknown. But this phenomenon seems to be ecologically
relevant, because other fungi (Geotrichum candidum,
Fusarium oxysporum, Gliocladium roseum)
did not show the same behaviour (Allan et al.,
1992). Idriella is a specialised weak
parasite of cereal and grass roots. It colonises
the naturally senescing root cortical cells before these
can be invaded by common soil saprotrophs, but it does
not cause significant damage to living root cells. In
this respect Idriella resembles Phialophora
graminicola (see Biology and control of take-all) and, like Phialophora, it can act
as a biological control agent, reducing or preventing
infection by several pathogens of cereal roots or stem
bases (the take-all fungus, eyespot fungus and Fusarium
culmorum which causes cereal foot rot). Presumably, the tropic responses of the
spores enable Idriella rapidly to colonise
senescing root cells and exploit their nutrients. Idriella
also produces a further batch of spores after it has
colonised the dying root cells (Figure G), and these
spores might be carried down the roots in percolating
water to provide general protection of the root zone. Idriella
is found commonly on cereal and grass roots in field
conditions, where it can be recognised by its production
of characteristic groups of darkly pigmented cells in the
dying tissues (Figures H, I).
|
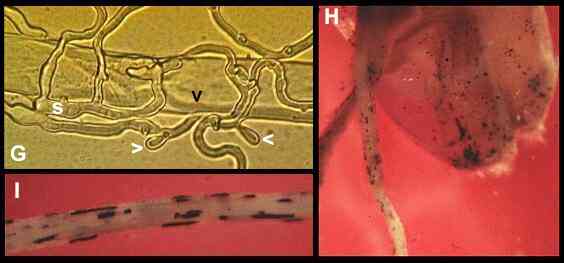
Figure
G.
Internal colonisation
(black arrowhead) of a dead wheat root hair from spores
(s) of Idriella bolleyi. The fungus has already
started to produce further spores (white arrowheads)
24-36 hours after colonising the dead root cell.
Figures
H, I.
Characteristic groups
of darkly pigmented resting cells of Idriella bolleyi
in the dead surface tissues of a wheat seed and young
wheat roots.
|
Spore
tropisms of Verticillium biguttatumVerticillium biguttatum is a
mycoparasite, specialised to invade the hyphae of other
fungi, which it exploits as a nutrient source. But it is
not aggressive like some other mycoparasites (see Pythium
oligandrum). Instead
it has a restricted host range and it starts its
parasitic phase as a biotroph, feeding
from the living hyphae of its hosts in much the same way
as the biotrophic plant pathogens. As shown in Figure J, the germinating
spores of V. biguttatum show a pronounced
tropism towards the host hyphae, leading to penetration
of the host and production of club-shaped haustoria
(nutrient-absorbing structures) within the living host
hypha. Having established this feeding relationship, Verticillium
grows over the host colony and produces many sporing
structures. It kills the older parasitised cells but
produces new haustoria at the advancing edge of the
infection.
|
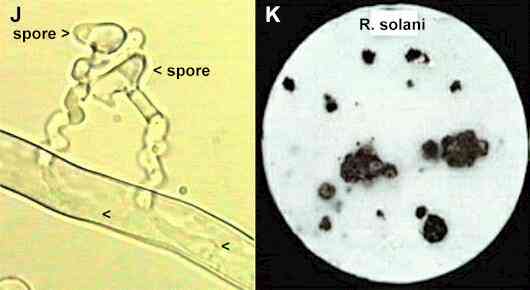
Figure
J.
Infection of a hypha
of Rhizoctonia solani from germinating spores of
Verticillium biguttatum. The germ-tubes showed
pronounced tropism, growing in spirals towards the host,
then they penetrated the Rhizoctonia hypha and
produced club-like haustoria (arrowheads). The
parasitised host hypha remained alive, with normal
protoplasmic streaming. Image taken from a videotaped
interaction on a thin film of water agar (van den Boogert
& Deacon, 1994).
Figure
K. The strain of Rhizoctonia
solani that causes black scurf of potatoes, growing
on sterile filter paper in laboratory culture. Most of
the fungal growth is inconspicuous, but after the filter
paper was colonised the nutrients in the hyphae were
mobilised to sites where the fungus produced large
sclerotia - the survival structures that cause the black
scurf symptoms on potato tubers.
|
V. biguttatum has attracted interest because its
main host is the important plant pathogen Rhizoctonia
solani - a fungus that causes seedling diseases of
many crops and that also causes "black scurf"
of potato tubers. This name refers to the black
crust-like sclerotia (resting structures) of R.
solani commonly seen on the surface of potato
tubers, reducing their market value. They also are
produced in laboratory culture (Figure K). Even a localised infection by V.
biguttatum can reduce the production of sclerotia on
colonies of Rhizoctonia (Figure L). Presumably
this is caused by the continuous withdrawal of nutrients
from the Rhizoctonia hyphal network. This raises
the possibility that Verticillium might be used
as a biocontrol agent of black scurf in commercial
conditions. But there are two major problems to overcome.
First, Verticillium can be inoculated onto the
"seed tubers" but does not spread efficiently
to the daughter tubers which are produced later in the
season. Second, Verticillium requires relatively
high temperatures (minimum about 13-15oC)
whereas Rhizoctonia can grow at much lower
temperatures and therefore becomes established early in
the season, before Verticillium can take effect.
|
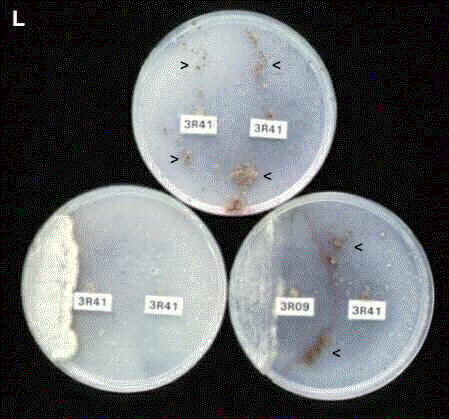
Figure L. Three plates of cellulose agar
inoculated with R. solani at two positions
(labelled).
Top plate:
inoculated with the same strain (3R41) at both positions.
Hyphae of the two colonies fused where they met [see
Figure R, below] and formed a single colony over the
whole plate. The fungus then produced clusters of
sclerotia (arrowheads) on both sides of the plate.
Bottom left plate:
as in the top plate, but spores of Verticillium
were streaked on one of the colonies. The mycoparasite
suppressed the production of sclerotia over the whole
agar plate.
Bottom right plate:
inoculated with two different strains of R. solani,
and spores of Verticillium were streaked on one
side of the plate. The two Rhizoctonia strains
are mutually incompatible - their hyphae fuse at the zone
of contact but the fused cells die (seen as a dark
crescent-shaped line on the agar plate). Verticillium
has suppressed the production of sclerotia on the
inoculated colony (3R09), not on the other colony (3R41).
Verticillium seems to suppress the production of
sclerotia by withdrawing nutrients from the Rhizoctonia
network, so a break in this network has restricted the
effect to one side of the plate [From van den Boogert
& Deacon, 1994].
|
Tropism of
rust germ tubesThe
rust fungi are major plant pathogens that establish
infections by producing haustoria in the host cells (see Biotrophic
Plant Pathogens). As a
prelude to this, these fungi often penetrate a leaf
through the stomatal openings, and they use contact
sensing to locate these sites. A classic example
of this is seen in the rust and powdery mildew fungi of
cereals. For example, when uredospores of Puccinia
graminis germinate on a cereal leaf the germ tubes
grow perpendicular to the rows of leaf cells. The same
behaviour is seen on inert replicas of cereal leaves
(Figure M), showing that the fungus responds to surface
topography and not to chemical signals. This behaviour is
thought to maximise the chances of locating a stoma,
because the stomata occur in lines (marked "s"
in Figure M) on cereal leaves and their positions vary in
the different lines.
|
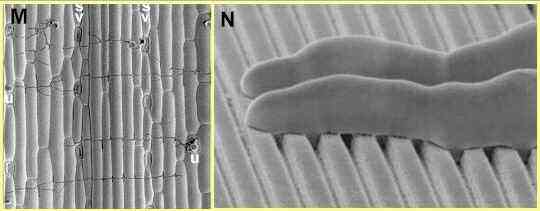
Figure M. Scanning electron micrograph of
germ tubes growing from uredospores (u) of Puccinia
graminis on an inert replica of a wheat leaf. Growth
of the germ tubes (seen as dark, narrow lines) is
orientated at right angles to the pattern of ridges and
grooves of the leaf surface cells. Lines of stomata (s)
occur at intervals across the leaf surface replica.
Figure N. Scanning electron micrograph of
germ tube tips of Puccinia graminis growing
perpendicular to precisely spaced ridges and grooves of a
polystyrene replica of a microfabricated silicon wafer.
The germ tubes are about 4 micrometres diameter; the
lower region of their tips is flattened against the
replica, presumably enabling them to sense the
topography.
[Images
supplied by Nick Read; see Read et al., 1992]
|
The rust fungi of dicotyledonous plants do not show this
behaviour, because the leaf surface cells and stomata of
dicotyledons are not arranged in rows. However, almost
all the rust fungi show another type of topographical
sensing - the uredospore germ tubes recognise stomata and
respond to them by producing a pre-penetration swelling
termed an appressorium. A remarkable insight into this behaviour was
achieved by Harvey Hoch & Richard Staples who used
the techniques of the microelectronics industry to
produce silicon wafers with precisely defined patterns of
ridges and grooves. The wafers were used as templates to
produce polystyrene replicas which were then inoculated
with uredospores. Working initially with the bean rust Uromyces
appendiculatus, it was found that germ tubes
produced appressoria when they encountered a single ridge
(or groove) of about 0.5 micrometre height, but showed
almost no response to heights above 1.0 micrometre. Other
rust fungi responded to different ridge heights (see
Allen et al., 1991), and these differences are
thought to reflect adaptations to different host plants,
for which the elevation of the stomatal guard cells may
provide the signal for production of an appressorium.
The germ tubes of cereal rust fungi
behave differently from the rest. They orientate at right
angles to a series of widely spaced ridges and grooves
(Figure N) which probably simulate the natural lines of
leaf surface cells. They do not form appressoria in
response to single ridges; instead, they require a series
of very closely spaced ridges, which perhaps signals to
them that they have located a stoma.
How do
external signals cause growth changes?
This question is relevant to all
the examples of tropism discussed so far, but the
evidence is best for topographical signalling, where at
least four factors have been identified.
1. Close adhesion is required
for sensing of topography, and this is achieved by
germ tube mucilage. Consistent with this, both
adhesion and contact sensing can be abolished by
treating the germ tubes with proteolytic enzymes
which destroy the proteinaceous component of the
adhesive.
2. The position of the Spitzenkörper seems to be
important, because germ tubes growing on
topographical surfaces have a "nose-down"
appearance (Figure N) and the Spitzenkörper is found
to be located close to the surface.
3. Electron
micrographs and the use of fluorescent dyes show that
there is a particularly high density of cytoskeletal
elements such as microtubules in the region of a germ
tube closest to a surface.
4. By using the
"patch clamp" technique on isolated
portions of fungal plasma membranes it has been shown
that the membrane contains stretch-activated calcium
channels.
Calcium is well known as
an important signalling ion in eukaryotic cells - it can
interact directly with cytoskeletal components and also
can act as a second messenger, transducing signals
received at the cell surface and leading to changes in
gene expression (see Jackson & Heath, 1993).
So, it is suggested that a
change in topography might cause a localised stress on
the fungal cell membrane, allowing the uptake of calcium
ions which then could act either directly on the
cytoskeleton to change the orientation of growth or
indirectly to alter gene expression leading to
differentiation.
Presumably the same
underlying mechanisms, but mediated by receptors in the
fungal membrane, could explain tropisms to chemical
factors.
|
PhototropismsThe spore-bearing structures of
several fungi are induced to develop by light (or
near-UV). In addition, some spore-bearing structures show
a phototropic response, bending towards a light source to
facilitate dispersal. This is often found in the dung
fungi (coprophilous fungi) which need to disperse their
spores onto the surrounding vegetation so that they will
be ingested by animals.
|
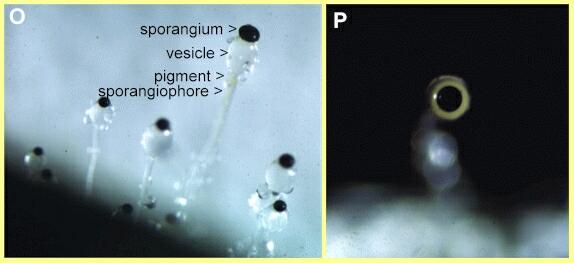
Figures O, P. Asexual spore-bearing structures of
Pilobolus (zygomycota) growing from a dung
pellet. The ring of flavonoid pigment which mediates the
phototropic response is faintly visible just below the
vesicle in Fig. O. The vesicle acts as a lens to focus
light on this pigment - an effect seen in Fig. P where
the large black sporangium is pointing towards the
camera, with the vesicle behind it.
|
Pilobolus
(Figures O, P) is a classic example of this because it
has a mechanism for shooting the whole sporangium clear
of a dung pellet. Its spore-bearing structures consist of
an erect hypha (sporangiophore) which is swellen into a
vesicle below the sporangium. At maturity the vesicle
wall ruptures and the vesicle sap squirts the sporangium
for a distance of several centimetres. Just below the
vesicle is a ring of flavonoid pigment. The vesicle acts
as a lens, focusing light on this pigment and causing the
sporangiophore to bend so that the sporangium is shot
towards the light source.A similar phototropic response is found in
the long sporangiophores of Phycomyces, another
genus of coprophilous fungi (Figure Q). In this case the
sporangiophore also acts as a lens but there is no
vesicle because the spores are released passively.
These phototropic
responses differ in one important way from the tropic
responses mentioned earlier, because the bending response
is achieved by differential wall extension behind the
growing tip, probably caused by a combination of
localised wall softening (involving wall lytic enzymes)
and localised wall growth or stretching. Enzymes with
flavin-containing prosthetic groups are quite common in
fungi, so a reduction of the flavin pigment by exposure
to light could easily change the activity of a
wall-associated enzyme.
|
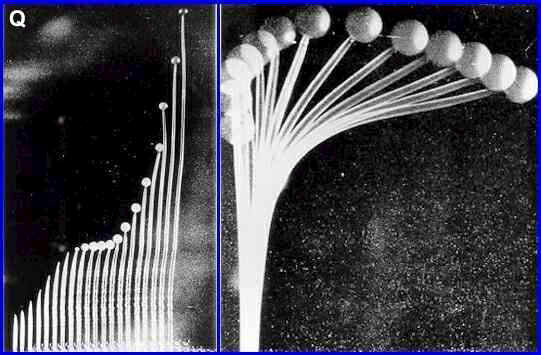
Figure Q. Sporangiophores
of the dung fungus Phycomyces (zygomycota). The
left hand image shows stages in elongation of the
sporangiophore to a height of 5 cm or more after the tip
has swollen to form the sporangium. This occurs by
extension of the wall beneath the sporangium. The right
hand image shows phototropic bending of the
sporangiophore over a period of 15 minutes. [Images based
on photographs in W. Shropshire, 1963; Physiological
Reviews 43, 38-67. Supplied by
Michael Carlile]
|
Sexual
tropismsThe
mating reactions of many fungi involve tropic responses
to bring two compatible mating types together. For
example, the production of sexual spores by the
zygomycota (see The Fungal Web) is achieved by the fusion of
aerial branches, which grow towards one another under the
influence of volatile hormones termed trisporic acids.
Similarly, many
basidiomycota undergo fusion (anastomosis) between the
normal vegetative hyphae of compatible strains as a
prelude to sexual development. These fusions involve
highly precise tropic responses, similar to those shown
in Figure R for hyphal fusions of Rhizoctonia solani.
The fact that these tropic responses (and hyphal fusions)
do not occur between different species indicates that the
signal molecules must be quite specific, but they have
not been identified yet (see Gooday & Adams, 1993).
|
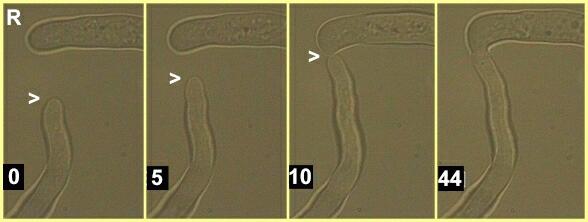
Figure R. Stages in anastomosis between
hyphae of two compatible strains of Rhizoctonia
solani. The times shown are minutes after the start
of video recording. Several reorientations of the apex of
the lower hypha (arrowhead) are seen to occur during this
sequence, and fusion is preceded by a directional
regrowth of the upper hypha (seen in the 10 minute frame)
so that the fusion occurs tip-to-tip. More than 30
minutes elapsed between hyphal contact (frame 3) and
complete fusion of the hyphal tips (frame 4). During this
time the walls of the hyphal tips dissolved to allow
cytoplasmic continuity.
|
Further reading
Reviews and
research papers:
P van den Boogert & JW
Deacon (1994) Biotrophic mycoparasitism by Verticillium
biguttatum on Rhizoctonia solani. European
Journal of Plant Pathology 100,
137-156.
ND Read, LJ Kellock, H Knight
& AJ Trewavas (1992) Contact sensing during
infection by fungal pathogens. pp. 137-172 in Perspectives
in Plant Cell Recognition (eds JA Callow &
JR Green). Cambridge University Press.
GW Gooday & DJ Adams (1993)
Sex hormones and fungi. Advances in Microbial
Physiology 34, 69-145.
EA Allen et al. (1991)
Appressorium formation in response to topographical
signals in 27 rust species. Phytopathology 81,
323-331.
RH Allan, CJ Thorpe & JW
Deacon (1992) Differential tropism to living and dead
cereal root hairs by the biocontrol fungus Idriella
bolleyi. Physiological and Molecular Plant Pathology
41, 217-226.
SL Jackson & IB Heath
(1993) Roles of calcium ions in hyphal tip growth. Microbiological
Reviews 57, 367-382.
GO TO FULL LIST OF PROFILES?
|