The take-all fungus, Gaeumannomyces graminis (ascomycota), is a major root-rot pathogen of cereals and grasses. It is most damaging to intensively grown wheat and barley crops, when the same crop is grown year after year in a site. It survives in the infected residues of one crop, then invades the roots of the following crop, progressively destroying the root system. In exceptional cases it can kill the whole crop; hence the name "take-all". G. graminis also causes a patch disease of turf grasses, especially of Agrostis species (the "bent grasses") which are used in high-quality turf such as on bowling greens and golf-course greens. Here we review briefly the biology of this pathogen, but focus mainly on two remarkable biological control systems that operate in natural field conditions and which can be exploited commercially. One of these involves the actions of antibiotic-producing fluorescent pseudomonads; the other involves competition from weakly parasitic fungi. |
||||||||||||||||||||||||
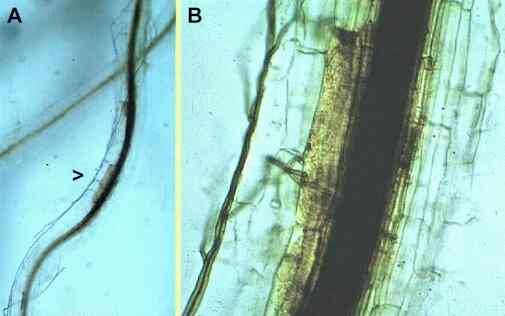
Figure A, low power micrograph of a wheat root infected by G. graminis, showing conspicuous vascular discoloration and dark runner hyphae of the fungus on the root surface. Figure B, close-up of the region shown as arrowhead in Fig. A, showing invasion of the root cortex, some browning reactions in the inner cortex and phloem, and intense blockage of the xylem by darkly pigmented vascular gels. |
||||||||||||||||||||||||
The fungus and the infection process The species Gaeumannomyces graminis is sub-divided into three varieties, which differ in minor morphological features but, more importantly, differ in pathogenicity:
All these fungi are specialised parasites of the grass family (Gramineae), and they grow along the surface of roots as darkly pigmented runner hyphae (Figure A). Branches that arise from these runner hyphae penetrate the root cortex at intervals, but their ability to infect depends on two factors - first, the nutrient reserves of the fungus (e.g. size of the organic food base from which it infects) and, second, the degree of host tissue resistance. In a successful infection by a pathogenic strain (see Figure B), the invading hyphae grow through the root cortex, invade and destroy the root phloem (sugar-transport system) and then invade the xylem (water-transport system), causing discoloration and blockage of the xylem vessels. The root is effectively cut off and decays below this point. The fungus then spreads progressively upwards towards the stem base, by producing runner hyphae and invading from these. Eventually it can colonise the stem base and kill the plant. Then the fungus survives as a saprotroph in the infected roots and stem bases or, in appropriate conditions, it can produce its sexual stage - a flask-shaped perithecium from which many elongated ascospores are ejected forcibly to disperse the fungus (Figures C, D). |
||||||||||||||||||||||||
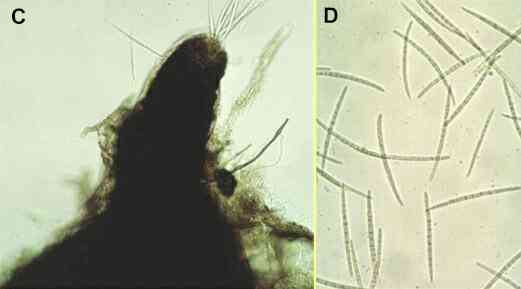
Figure C, the neck region of the perithecium of G. graminis, showing needle-shaped ascospores emerging from the apical pore where the perithecium was crushed. Figure D, individual ascospores, each about 100 micrometres long and with several septa (cross walls). |
||||||||||||||||||||||||
Take-all patch disease of turf grasses Take-all patch disease of turf grasses (Figures E, F) is caused by G. graminis var. avenae. However, most grass species are quite resistant to take-all infection, and only the "bent grasses" (Agrostis species) are seriously affected. Even these grasses are seldom diseased in field sites, although they are extremely susceptible to attack in glasshouse tests. The reason is that they are protected by a natural biocontrol system, and disease occurs only when the natural biocontrol agents are eliminated by management practices. Specifically, the take-all patch disease is seen when:
In all these circumstances, the pathogen infects from air-borne ascospores, which become washed into the soil and initiate infection of roots. In all normal soils the ascospores do not have sufficient nutrient resources to infect roots. This infection from air-borne ascospores explains why the disease occurs in patches, each representing a separate focus of infection. But even these disease patches only spread for two or three years before they become quiescent, probably because the normal biocontrol agents of grass roots become re-established and halt the spread of disease. |
||||||||||||||||||||||||
Take-all patch disease of bent-grass turf (Agrostis species). Figure E shows part of a golf-course green with numerous spreading disease patches. The grass has been killed at the advancing (white) margins of these patches. Figure F shows a close-up of disease patches, where the fine turf (Agrostis spp.) has been killed and the centres of the patches have been invaded by coarser-leaved grasses and weeds that affect the playing qualities of the turf. |
||||||||||||||||||||||||
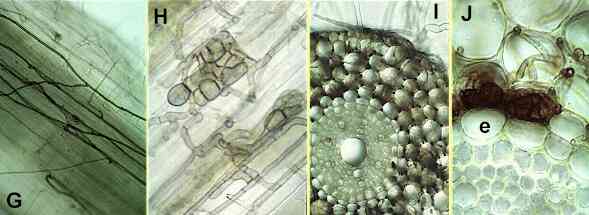
The natural control agents Several fungi and bacteria might contribute to biocontrol of take-all patch disease, but most attention has focused on the weakly parasitic fungi of grass roots. One of these fungi, Phialophora graminicola, is extremely common on the roots of turf grasses in Britain and continental Europe, and in glasshouse conditions it is a highly effective biocontrol agent of take-all. It grows on the surface of roots as dark runner hyphae (Figures G-J), but it is only a weak parasite, unable to invade the living root cortical cells and, crucially, unable to penetrate the root endodermis - the layer of suberised cells that surrounds the phloem and xylem. Thus, this fungus does not enter the vascular system and does not cause disease. When its attempted penetration of living root cells is halted by host resistance factors, Phialophora produces characteristic groups of swollen, pigmented cells (Figures H, J) which provide a simple way of identifying its presence in roots. G. graminis var graminis (another weak parasite) plays a similar role in controlling the take-all pathogen on grasses in Australia and other warm countries. |
||||||||||||||||||||||||
Figures G-J. Phialophora graminicola, a
weak parasite of cereals and turf grasses. G, dark runner hyphae on the surface of a
cereal or grass root. H, groups of darkly pigmented cells
produced by the fungus when its attempted penetration of
host cells is halted. I, cross section of an older root,
in which the root cortical cells have died and been
colonised by P. graminicola, but the fungus has
been stopped at the root endodermis. J,
close-up of part of Fig I, showing Phialophora
hyphae in the root cortex (upper part of the Figure), but
no invasion of the endodermis (e). |
||||||||||||||||||||||||
The involvement of these fungi helps to explain the specific conditions in which take-all patch disease occurs. For example, the table below shows that Phialophora graminicola is abundant in turf of relatively high pH (pH 6 and above), but repeated applications of ammonium fertiliser can lower the turf pH to a level (about pH 4) where Phialophora is eliminated. At such low pH levels the grass itself begins to suffer, and when it is limed heavily to correct for this the take-all fungus can infect from ascospores. Eventually, Phialophora becomes re-established and prevents the disease patches from spreading further. In short, take-all patch disease is caused by mismanagement of turf pH, and by other practices which fail to conserve the natural biocontrol agents.
|
||||||||||||||||||||||||
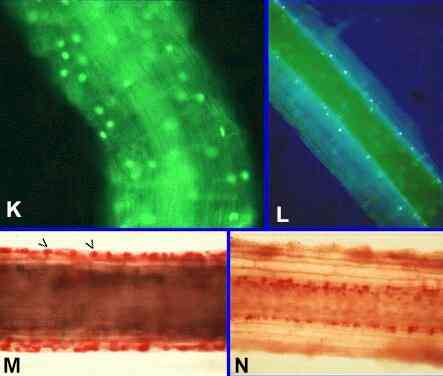
Mode of action of P. graminicola Phialophora and other weak parasites like G. graminis var graminis have no effect on the take-all fungus in laboratory culture - they do not damage or destroy the pathogen hyphae (compare this with the effects of Pythium oligandrum or Phlebiopsis gigantea) and they do not produce inhibitory compounds (compare this with Agrobacterium radiobacter). So there are only two possible mechanisms to explain their roles in biocontrol: they might activate the host defences against the pathogen or they might compete for resources - a form of biocontrol termed competitive niche exclusion. There is strong evidence that competition plays at least some role. The weak parasites grow in the same way as the pathogen on the root surface (compare Figures A or B with Figure G) and, presumably, compete for the same resources. The key to understanding this came from the discovery that cereal and grass roots show an early natural senescence of the root cortex (Figures K-M). It begins in the root epidermis soon after the root hairs die, and it progresses inwards with increasing distance (and age of the root region) behind the root tip. Meanwhile, the root tips continue to extend into fresh zones of soil, and the older root regions (with senescent cortex) have a predominantly transport function - the vascular tissues remain alive, protected by the living root endodermis. Phialophora and other weak
parasites (e.g. Idriella
bolleyi) are
specifically adapted to exploit this natural root
cortical death. They rapidly colonise the root cortical
cells as these begin to senesce, and before the cells can
be colonised by soil saprotrophs. In doing so, these
fungi can prevent the invasion of G. graminis
from ascospores, which have low nutrient reserves,
because G. graminis then depends on the
senescing cortical cells to build up its nutrient
reserves for infection. |
||||||||||||||||||||||||
Figures K-M. Natural root cortical senescence of
cereals and grasses. This natural senescence can be shown
in several ways, including nuclear staining with acridine
orange (K, L) and cytoplasmic staining with neutral red
(M, N). Figure K. Part of a wheat root about 1 week
old, stained with acridine orange and viewed by
fluorescence microscopy. Nuclei are seen in all cell
layers of the cortex, evidenced by fluorescence of
acridine orange intercalated in the DNA. Fig. L. Part of a wheat root in a region 3 weeks
old, showing nuclei in the inner cortex, but the outer
cortical cell layers are anucleate and (right-hand side
of root) have been shed. Fig. M.
Young region of a wheat root treated with neutral red and
strong salt solution. Neutral red stains the cytoplasm,
while the salt solution plasmolyses the living cells,
which are seen as contracted, densely stained protoplasts
(arrowheads). Fig. N. Older region of a similar root,
showing that living cells are present only in the inner
cortex. |
||||||||||||||||||||||||
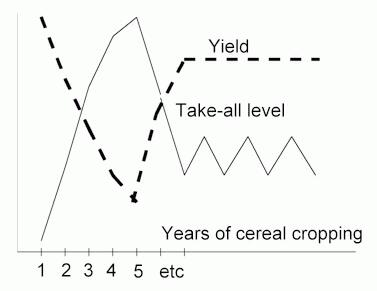
Take-all of cereal crops A different type of biocontrol has been identified in continuously grown cereal crops. It is termed take-all decline or take-all suppressiveness. As shown in Figure O, the level of take-all infection builds up progressively in the first few years of cereal monocropping, because the inoculum in the residues of one crop serves as a food base for infection of the next. But the disease level reaches a peak in the third to fifth year and then declines spontaneously to a level that gives economically acceptable yields. Fluorescent pseudomonads are thought to be the main organisms responsible for this dramatic disease decline. Recent work (see Further Reading) implicates two specific sub-groups of fluorescent pseudomonad - those that produce phenazine-type antibiotics (Phe) and those that produce 2,4-diacetylphloroglucinol (Phl). |
||||||||||||||||||||||||
Figure O. Diagram to illustrate take-all
decline in continuous cereal cropping. The
disease level builds to a peak in the third to fifth year
(depending on climatic and field conditions) then
spontaneously declines to a lower, relatively stable
level. Grain yields respond accordingly. |
||||||||||||||||||||||||
The populations of Phl strains, in particular, are markedly higher in suppressive than in non-suppressive soils, suggesting that these bacteria are favoured by the build-up of disease, which they subsequently help to control. The Phl strains have been grown in laboratory conditions and applied to wheat seeds which are then sown in soil. They colonise the emerging wheat roots, attaining high population levels, and the Phl antibiotic can be detected in the root zone by high-performance liquid chromatography. Also of interest, naturally occurring Phl-producing pseudomonads have been implicated in suppression of other root diseases in various crops in Europe and the USA. Consistent with this, 2,4-diacetylphloroglucinol is a broad-spectrum antibiotic (also toxic to plant roots if it is over-produced in the root zone). The gene coding for biosynthesis of this antibiotic has been identified in Pseudomonas fluorescens and used as a probe to show that it is a highly conserved gene among fluorescent Pseudomonas strains from many parts of the world. The hope is that these antibiotic-producing bacteria might be applied commercially as seed coatings, to prevent or reduce take-all or other root diseases. |
||||||||||||||||||||||||
Further reading
|
||||||||||||||||||||||||