2. Rust
fungiThe
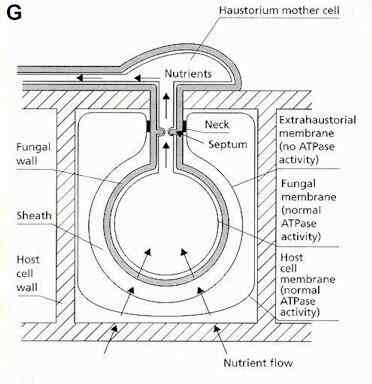
infection behaviour of rust fungi is broadly similar to
that of the powdery mildews, involving nutrient
absorption by haustoria to support abundant sporulation
for epidemic spread. These fungi also get their name from
the characteristic sporing stage - in this case the
(usually) rust-coloured uredospores which develop in
pustules where the fungus erupts through the plant
surface.

Figure H. Wheat leaf infected by the rust
fungus, Puccinia graminis var tritici,
showing individual lesions (light coloured haloes on the
leaf) with pustules of uredospores in their centres.
[Image taken by placing an infected leaf on a flat-bed
scanner]
|
The life cycle of rust
fungi (basidiomycota, related to the toadstool-producing
fungi) is often more complex than that of powdery
mildews, because some rust fungi need two different types
of host to complete their cycle. These hosts are termed
the main host and the alternate
host. For
example, Puccinia graminis var. tritici
has wheat as its main host and barberry plants (Berberis
species) as its alternate host. There is a
correspondingly large number of sporing stages - up to 5
in some cases, as shown
below.
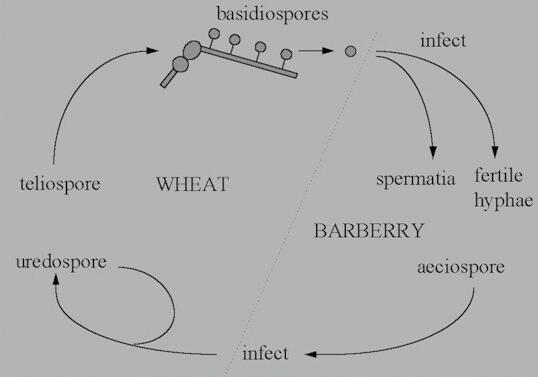
Figure I. Life cycle of Puccinia
graminis var tritici.
|
On wheat:
- P. graminis
produces uredospores from a bed
of tissue that erupts through the leaf or stem
surface (Figures J, K). These
uredospores can reinfect another wheat plant (see
Fungal tip growth), leading to multiple
cycles of infection during the cropping season.
They are binucleate spores,
containing nuclei of different mating types, and
they germinate to produce hyphae that have 2
nuclei in each hyphal cell. In this condition,
the fungus is termed a dikaryon
(i.e. with two nuclear types).
- Near the end of the
growing season, the same pustules produce a
different type of spore - the teliospore,
which consists of two cells with heavily
thickened and darkly pigmented walls (Figure
L). The teliospores also are dikaryons,
with two nuclei in each cell.
- The teliospores
overwinter, and in spring the nuclear pairs fuse
to form diploid nuclei. This is followed
immediately by meiosis, then the spore germinates
from each cell to form a short hypha that
produces 4 uninucleate, haploid basidiospores
(see Figure I).
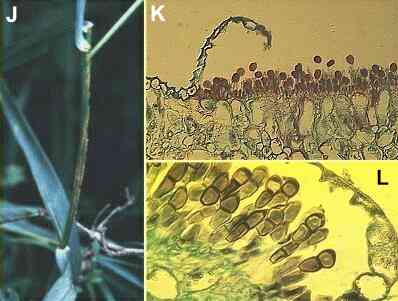
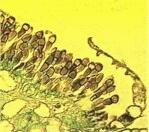
Figures
J-L. Puccinia
graminis on the cereal host. (J)
Pustules of uredospores on a cereal stem. (K)
Section of a leaf showing eruption of uredospores through
the leaf epidermis (stained with safranin). (L)
Section of a leaf later in the season, showing
teliospores in place of the uredospores that were
produced earlier.
|
On barberry:
- The basidiospores
can only infect a barberry plant. They give rise
to haploid hyphae of different mating types,
which grow through the barberry leaf. These
hyphae produce flask-shaped sexual structures
termed spermogonia on the upper
surface of the barberry leaf (Figures M and N). Small "male"
sexual spores (spermatia) are
formed within the spermogonia, and
"female" flexuous hyphae
project from the neck of the spermogonium, among
the stiffer hairs (arrowhead in Figure N).
- Fertilisation of
flexuous hyphae by spermatia of a different
mating type is brought about by insects. Then the
nuclei pair in the hyphae, forming a dikaryon
which gives rise to sporing pustules on the lower
surface of the barberry leaf (Figures O
and P).
- The spores from these
pustules are termed aeciospores.
They can only infect a cereal host, thereby
completing the life cycle.
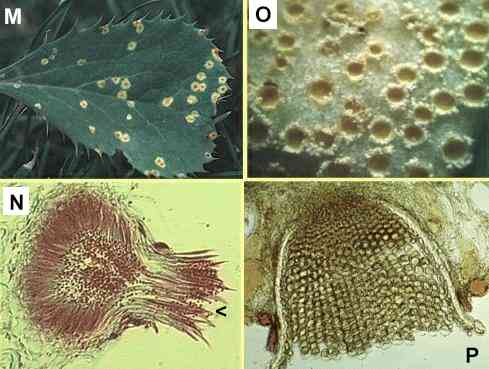
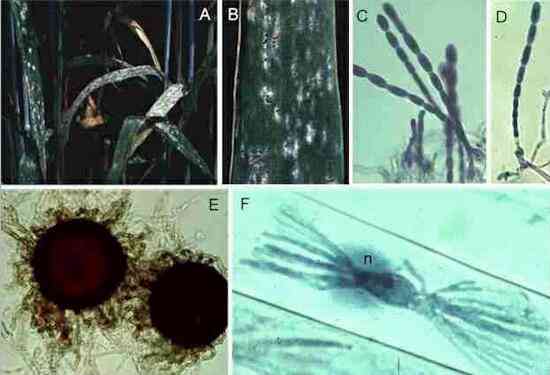
Figures
M-P. Puccinia
graminis on the alternate host, barberry. (M)
Small lesions on the upper surface of a barberry leaf,
with spermogonia in their centres. (N)
Section of a spermogonium, showing the minute spermatia
(male sexual cells) and the position (arrowhead) where
flexuous (female) hyphae arise. (O)
Close-up of lower surface of the leaf, showing cup-shaped
pustules of aeciospores. (P) Cross
section of a leaf showing the aeciospores developing in
tightly packed chains from a pad of fungal tissue.
|
Some
common rust fungiRust
fungi are remarkably common on both crop plants and wild,
native plants. On crops they cause serious economic
damage, necessitating the use of fungicides. Although Puccinia
graminis (black stem rust of cereals) is most
important in the USA, Puccinia striiformis
(yellow rust) and P. recondita (brown rust) are
more important on cereals in Britain.
Several other rusts are
common in Britain.
- Phragmidium
violaceum produces pustules of violet
teliospores on the leaves of blackberry bushes (Rubus
fruticosus) (Figures Q, R). The stalked teliospores of this
fungus are highly distinctive (R).
There is no alternate host in this case, only the
main host.
- Puccinia
punctiformis (thistle rust)
is also commonly seen (Figure S). It grows systemically in the thistle
Cirsium arvense, overwintering as
mycelium in the rootstock, and producing
chocolate-brown aeciospores. This fungus also has
no alternate hosts.
- Another common
species is birch rust, Melampsoridium
betulinum, which forms abundant uredospores
(Figure T) and aeciospores on birch
leaves. Larch trees are the alternate host of
this fungus.
- Further common
species include mint rust, groundsel rust (Coleosporium
tussilaginis; Figures U, V), dandelion rust, hollyhock
(mallow) rust and snapdragon (Antirrhinum)
rust.
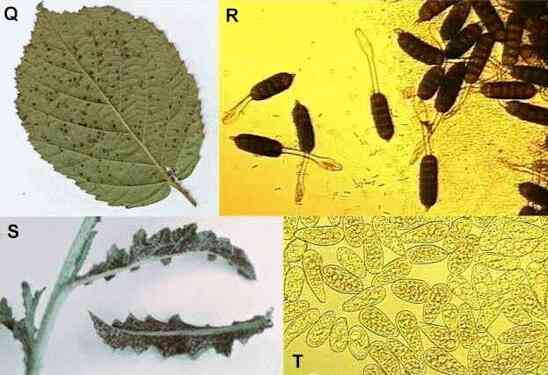
Figure Q-R.
Blackberry rust,
showing pustules of aeciospores on the leaf surface (Q) and the stalked, multicellular aeciospores
under a microscope (R).
Figure S. Thistle
rust. Figure T. A mass of uredospores of birch
rust, each about 30 micrometers long and easily impacted
onto leaf surfaces during wind-dispersal.
|

Figures
U,V. Groundsel (Senecio
vulgaris), a common weed of open ground. U,
whole plant (about 15 cm tall) with rust infection at the
base; V, close-up of base, showing
uredospore pustules of Coleosporium tussilaginis on
the stem and leaves.
|
Further reading.Books:
JW Deacon (1997) Modern
Mycology. Blackwell Scientific, Oxford.
Websites:
An excellent site from
a public-service disease diagnostic lab in USA: Oklahoma State University Diagnostic
Laboratory
GO
TO FULL LIST OF PROFILES?
|