|
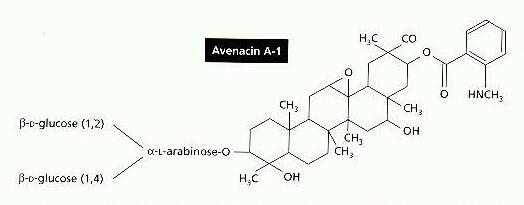
Saponins are membrane-active agents which lyse red blood cells or other wall-less cells. They are produced by several plants and are thought to act as resistance compounds against plant pathogens. In some cases such as avenacin A, discussed below, the saponins occur as pre-formed resistance compounds in the plant tissues. In other cases they are produced rapidly from inactive precursors when the plant tissues are damaged. The typical structure of a saponin is shown in Figure A, for one of the main saponins in the roots of oats (Avena species). This molecule, termed avenacin A, has a large lipophilic region and a short side chain of sugar residues which interact with non-lipid components. For this reason saponins are soap-like, and they are thought to cause cell disruption by interaction with sterols in the cell membrane.
The roles of saponins as plant resistance factors have been demonstrated quite conclusively for the take-all fungus of cereals, Gaeumannomyces graminis (see Biology and Control of Take-all). This fungus has two pathogenic varieties - var. tritici which infects wheat but cannot infect oats, and var. avenae which infects both wheat and oat crops. The difference in host range of these two varieties is matched by their ability to grow on oat root extracts in vitro - var. tritici is inhibited by even low concentrations of oat extract, whereas var. avenae is inhibited initially but rapidly overcomes this by detoxifying the saponin. It produces a glycosidic enzyme termed avenacinase, which cleaves some of the terminal sugars from avenacin, rendering the toxin inactive. This was discovered originally by EM Turner and reported in a series of papers (see Turner, 1960). It has been confirmed recently by targetted disruption of the avenacinase gene of G. graminis var avenae - strains of the pathogen with an inactivated avenacinase gene are unable to infect oats (Osbourn, 1996). |
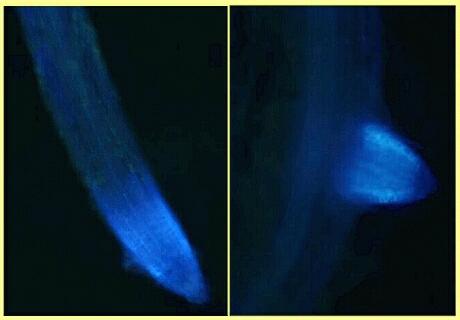
The roots of oats, and of the related wild grass Arrhenatherum elatius (false oat-grass), have a profound effect on fungal zoospores. The zoospores are attracted to the root tip region by chemotaxis but they are immobilised when they approach the root surface and they subsequently lyse. This is probably caused by avenacin, diffusing more slowly from the roots than the chemoattractant molecules. So, in effect, oat roots cause the zoospores to home-in on the toxin. This raises the possibility, as yet unexplored, that oats or oat tissues might be used as a simple means of controlling zoosporic fungi in commercial cropping systems. Avenacin is a naturally fluorescent compound, so its distribution in oat root tissues can be visualised under a fluorescence microscope (Figure B). It is found in large quantities in the root tips and in the newly emerging tips of root laterals, but in only low levels in the rest of the root system.
|
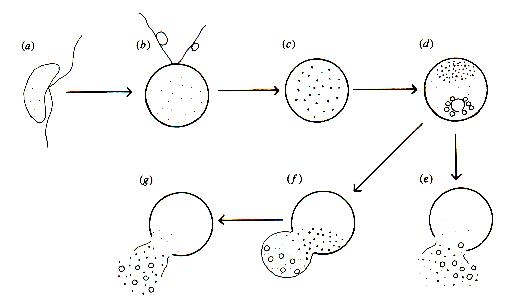
This effect of oat root extracts can be paralleled by pure saponins, such as beta-aescin, which is extracted from the leaves of horse chestnut trees (Aesculus hippocastanum) and is used commercially to stabilise the "head" of some beers. Figure C shows the observed changes in zoospores treated with beta-aescin.
|
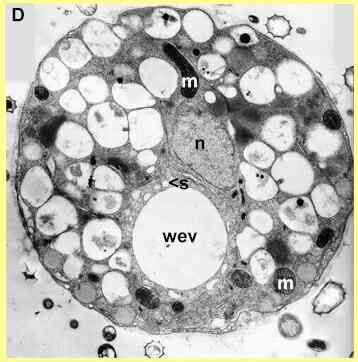
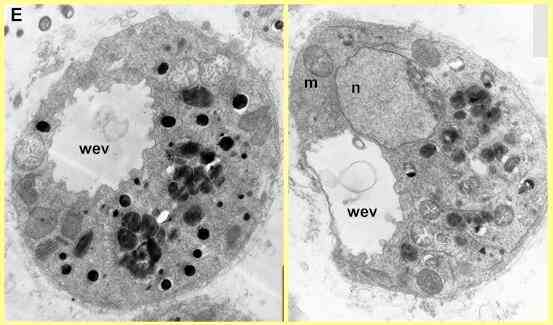
Electron microscopy shows that there is a rapid and generalised breakdown of the cellular organisation when zoospores are exposed to saponins. Figure D shows an untreated (control) cell in the early stages of encystment. The nucleus has migrated to a central position, and the cell contains numerous vacuoles and mitochondria. There is also a conspicuous water-expulsion vacuole (WEV), consisting of a large central vacuole and smaller surrounding vacuoles (one marked by 's'). At regular intervals (7-10 sec) the central vacuole expels its contents and disappears; then the surrounding vacuoles coalesce to produce a new central vacuole. This process continues until the cyst wall has developed; then the WEV disappears.
In contrast to this normal organisation, Figure E shows two zoospores treated with a saponin. The water-expulsion vacuole has an irregular, convoluted appearance, because the surrounding vacuoles have fused with the central vacuole. Many of the mitochondria have a degenerate appearance, and the normal vacuoles of the cells have aggregated, with electron-dense contents. Cells like this do not produce a cyst wall so they are destined to die.
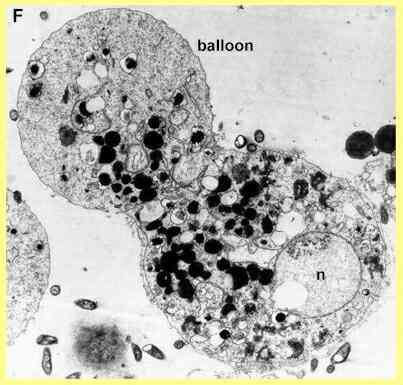
Light-microscopy of Pythium zoospores treated with beta-aescin (Sion W Jones & Deacon, unpubl.) showed that the water-expulsion vacuole swells to occupy a large volume of the cell and then discharges in a 'violent' manner, releasing some cell contents as well as the contents of the WEV. This could indicate a disruption of the normal process by which the membrane of the WEV fuses with the cell membrane. The process occurred two or three times, at irregular intervals, before the cells lysed from the region near the WEV. Some cells also produced a balloon-like swelling from this region (see Figure F) before they lysed.
|
Further reading
|