Agrobacterium tumefaciens causes crown gall disease of a wide range of dicotyledonous (broad-leaved) plants, especially members of the rose family such as apple, pear, peach, cherry, almond, raspberry and roses. A separate strain, termed biovar 3, causes crown gall of grapevine. The disease gains its name from the large tumour-like swellings (galls) that typically occur at the crown of the plant, just above soil level. Although it reduces the marketability of nursery stock, it usually does not cause serious damage to older plants. Nevertheless, this disease is one of the most widely known, because of its remarkable biology. Basically, the bacterium transfers part of its DNA to the plant, and this DNA integrates into the plant’s genome, causing the production of tumours and associated changes in plant metabolism.
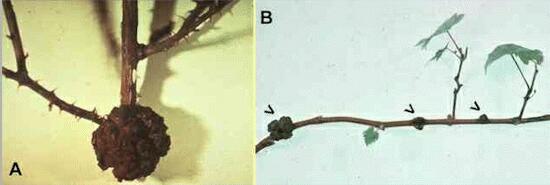
Figure A. Large gall formed at the base of the stem of a rose bush. Figure B. A series of galls (arrowheads) along a branch of a grapevine. |
|||||||||||||||||||||||||
The unique mode of action of A. tumefaciens has enabled this bacterium to be used as a tool in plant breeding. Any desired genes, such as insecticidal toxin genes (see Bacillus thuringiensis) or herbicide-resistance genes, can be engineered into the bacterial DNA and thereby inserted into the plant genome. The use of Agrobacterium not only shortens the conventional plant breeding process, but also allows entirely new (non-plant) genes to be engineered into crops. The story of Agrobacterium goes even further than this, making it one of the most interesting and significant bacteria for detailed study. For example, there is a highly effective biological control system for this disease - one of the first and most successful examples of biological control of plant disease. Here we consider three major aspects of this intriguing disease:
|
|||||||||||||||||||||||||
The bacterium and its plasmids A. tumefaciens is a Gram-negative, non-sporing, motile, rod-shaped bacterium, closely related to Rhizobium which forms nitrogen-fixing nodules on clover and other leguminous plants. Strains of Agrobacterium are classified in three biovars based on their utilisation of different carbohydrates and other biochemical tests. The differences between biovars are determined by genes on the single circle of chromosomal DNA. Biovar differences are not particularly relevant to the pathogenicity of A. tumefaciens, except in one respect: biovar 3 is found worldwide as the pathogen of gravevines. But this is almost certainly because biovar 3 has been spread around the world in vegetative cuttings of vines, not by natural mechanisms. Most of the genes involved in crown gall disease are not borne on the chromosome of A. tumefaciens but on a large plasmid, termed the Ti (tumour-inducing) plasmid. In the same way, most of the genes that enable Rhizobium strains to produce nitrogen-fixing nodules are contained on a large plasmid termed the Sym (symbiotic) plasmid. Thus, the characteristic biology of these two bacteria is a function mainly of their plasmids, not of the bacterial chromosome. [A plasmid is a circle of DNA separate from the chromosome, capable of replicating independently in the cell and of being transferred from one bacterial cell to another by conjugation. Plasmids encode non-essential functions, in the sense that a bacterium can grow normally in culture even if the plasmid is lost.] The central role of plasmids in these bacteria can be shown easily by "curing" of strains. If the bacterium is grown near its maximum temperature (about 30oC in the case of Agrobacterium or Rhizobium) then the plasmid is lost and pathogenicity (of Agrobacterium) or nodule-forming ability (of Rhizobium) also is lost. However, loss of the plasmid does not affect bacterial growth in culture - the plasmid-free strains are entirely functional bacteria. In laboratory conditions it is also possible to cure Agrobacterium or Rhizobium and then introduce the plasmid of the other organism. Introduction of the Ti plasmid into Rhizobium causes this to form galls; introduction of the Sym plasmid into Agrobacterium causes it to form nodule-like structures, although they are not fully functional. Studies such as these
raise many interesting and challenging questions about
the nature of bacteria. For example, what does the name
of a bacterial species or genus really mean, if the
organism can change so drastically by loss or gain of a
non-essential plasmid? And how much gene exchange occurs
my means of plasmids and other mobile genetic elements
within natural populations? |
|||||||||||||||||||||||||
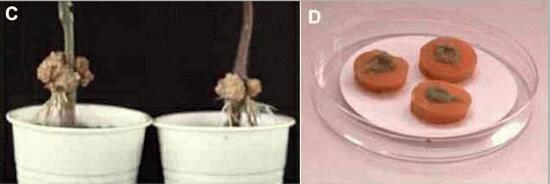
The infection process Agrobacterium tumefaciens is found commonly on and around root surfaces - the region termed the rhizosphere - where it seems to survive by using nutrients that leak from the root tissues. But it infects only through wound sites, either naturally occurring or caused by transplanting of seedlings and nursery stock. This requirement for wounds can be demonstrated easily in laboratory conditions. For example, Figure C shows the bases of two young tomato plants where a drop of A. tumefaciens bacterial suspension was placed on the stem and a pin prick was then made into the stem at this point. The photograph was taken 5 weeks later. Figure D shows another laboratory assay, where bacterial suspension was added to the surface of freshly cut carrot disks. After 2 weeks the young galls (green-coloured) developed from the meristematic tissues around the central vascular system.
|
|||||||||||||||||||||||||
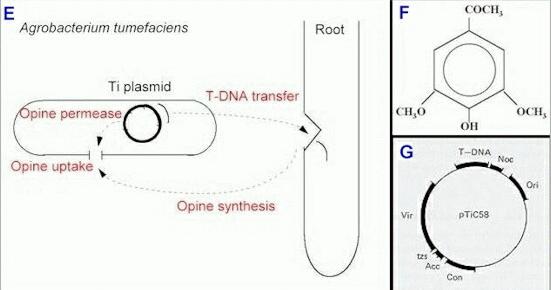
In natural conditions, the motile cells of A. tumefaciens are attracted to wound sites by chemotaxis. This is partly a response to the release of sugars and other common root components, and it is found even in plasmid-cured strains. However, strains that contain the Ti plasmid respond even more strongly, because they recognise wound phenolic compounds such as acetosyringone (Figure F) which are strongly attractive at even very low concentrations (10-7 Molar). Thus, one of the functions of the Ti plasmid is to code for additional, specific chemotactic receptors that are inserted in the bacterial membrane and enable the bacterium to recognise wound sites. Acetosyringone plays a further role in the infection process, because at higher concentrations (about 10-5 to 10-4 Molar) than those that cause chemotaxis it activates the virulence genes (Vir genes) on the Ti plasmid (see Figure G). These genes coordinate the infection process and, in particular:
As shown diagrammatically in Figure E, the excised T-DNA is released by the bacterium and enters the plant cells, where it integrates into the plant chromosomes and dictates the functioning of those cells. The actual mechanism of transfer is still unclear, but it seems to require a conditioning process, perhaps mediated by the production of cytokinins (plant hormones) by the bacterium. The tzs (transzeatin) gene on the Ti plasmid codes for the hormone (Fig. G).
Figure E. Overview of infection of a plant
wound site by Agrobacterium tumefaciens. The Ti
plasmid codes for a nutrient-uptake protein (opine
permease) that inserts in the bacterial cell membrane.
The plasmid also copies and excises part of its DNA,
which enters the plant cells and causes them to produce
opines. |
|||||||||||||||||||||||||
It is important to note that only a small part of the plasmid (the T-DNA) enters the plant; the rest of the plasmid remains in the bacterium to serve futher roles. When integrated into the plant genome, the genes on the T-DNA code for:
The plant hormones upset the normal balance of cell growth, leading to the production of galls and thus to a nutrient-rich environment for the bacteria. The opines are unique amino acid derivatives, different from normal plant products, and the agrocinopines similarly are unique phosphorylated sugar derivatives. All these compounds can be used by the bacterium as the sole carbon and energy source, and because they are absent from normal plants they provide Agrobacterium with a unique food source that other bacteria cannot use. Different strains of A. tumefaciens contain different types of Ti plasmid which code for production of different types of opines. One of the most common types of Ti plasmid (found in strain C58 of A. tumefaciens; Fig. G) codes for production of nopaline (structure shown below), and for agrocinopine A. The part of the plasmid that remains in the bacterium codes for the uptake and catabolism of these compounds (the Noc gene and the Acc gene shown in Fig. G). The other common type of Ti plasmid codes for synthesis of octopine and agropine. The significance of this difference will be clear when we discuss biological control of crown gall.
To end this account of the disease process, we should return to a question raised earlier: how much genetic exchange occurs between bacteria in natural conditions? When Agrobacterium is isolated from the root surface of plants in natural or cropping environments, the majority of strains (90% or more) are found to be non-pathogenic - even when the isolations are made from diseased plants. These non-pathogenic strains have traditionally been called by the species name Agrobacterium radiobacter. So we must conclude that Agrobacterium is essentially a rhizosphere inhabitant, and only a small proportion of strains are pathogenic (containing the Ti plasmid). Incidentally, the same is true of Rhizobium - most strains isolated from the root zone are incapable of nodulating plants. In many ways this makes sense biologically: the bacterium is, basically, a rhizosphere inhabitant because pathogenic strains of Agrobacterium could only respond rapidly to wound sites if there were an established population in the root zone. But the Ti plasmid is a conjugative plasmid - it can be transferred from one cell to another, under the control of the Con region (Figure G). In laboratory conditions, this conjugative transfer is strongly promoted by the presence of nopaline, so it seems that the pathogenic strain creates the conditions (nopaline production from infected wound sites) that enable it to transfer its plasmid to other strains in the rhizosphere. |
|||||||||||||||||||||||||
Biological control of crown gall In general, bacterial diseases of plants are very difficult to control owing to the lack of effective chemicals. Antibiotics could be used, but they are expensive and, in any case, the compounds that are valuable for human therapy are not allowed to be used in agriculture. The most effective alternative is the use of copper, which is potentially phytotoxic. However, for the nopaline-producing strains of A. tumefaciens there is a highly effective biocontrol system, discovered and developed by Allan Kerr in Australia. It has been used in Australia since 1973 - the first commercial biological control agent for any plant disease. It is now used world-wide, and marketed by several companies under a range of trade names (e.g. "Galltrol"). See Crown Gall Disease of Nursery Crops (Oregon State University) (not on this server) Kerr discovered this biocontrol system by isolating non-pathogenic strains of Agrobacterium radiobacter from disease sites and testing their ability to compete with pathogenic strains in mixed inoculations. Several non-pathogenic strains helped to reduce infection, but one strain in particular, A. radiobacter strain K84, completely prevented disease when added to wound sites at a 1:1 ratio with cells of A. tumefaciens. This strain is the one that is marketed globally. It is supplied commercially on agar plates or in a peat substrate, and it is used by suspending the bacterial cells in water, then dipping seeds, seedlings or cuttings in this suspension before planting. It acts only as a preventative treatment, not to cure infections, so it is applied at a high population level to protect any wound sites against pathogenic invasion. |
|||||||||||||||||||||||||
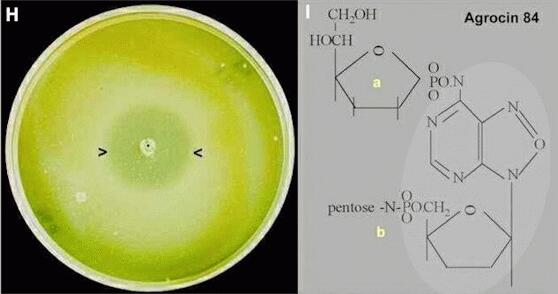
Mode of action of strain K84 As shown in Figure H, the high biocontrol efficiency of A. radiobacter strain K84 compared with other strains is related to production of an inhibitor in laboratory culture. Only strains of A. tumefaciens containing a nopaline-type Ti plasmid are found to be inhibited in plate assays, and only these strains are controlled effectively by strain K84 in plant tests (see Figure J, below). Fortunately, nopaline-utilising strains are the more common pathogens in many agricultural and horticultural sites. Pathogenic strains with plasmids of the octopine-agropine type are not inhibited, and nor are unrelated bacteria. These findings preclude a role for a "conventional" broad-spectrum antibiotic, but they are typical of the actions of bacteriocins - compounds that are produced by several bacteria (e.g. Escherichia coli) and that act specifically on other strains of the same or closely related species. However, unlike most bacteriocins, which are proteinaceous, the bacteriocin produced by strain K84 was found to have a unique type of structure (Figure I) and was termed agrocin 84. It is a fraudulent adenine-type nucleotide (the lighter shaded part of Fig. I) with two sugar derivatives attached to it: (a) a 6-carbon glucofuran phosphate and (b) a methylated pentanamide (details not shown fully). [ME Tate et al., 1979; Nature 280, 697-699].
Figure H. Agar plate stab-inoculated in the centre with A. radiobacter strain K84 and incubated for 24 hours before the bacterium was killed with chloroform vapour. Then a top layer of cooled agar containing A. tumefaciens was poured over the plate (the overlay plate technique). Growth of the pathogen is inhibited in a wide zone (arrowheads) around the spot where strain K84 had grown. [Note: both bacteria produce a creamy-white growth; the plate appears yellow because the image was photo-processed to accentuate the inhibition zone]. Figure I. Structure of agrocin 84. |
|||||||||||||||||||||||||
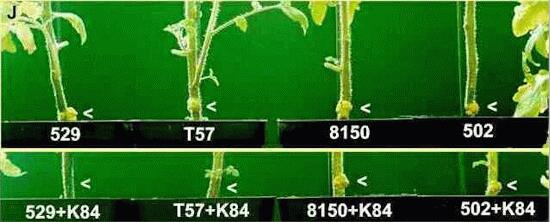
The two pathogenic strains 529 and T57 have a Ti plasmid coding for nopaline production; they are controlled by strain K84. The strains 8150 and 502 have Ti plasmids coding for other opines and are not controlled by strain K84. [Figures H and J are from material produced as laboratory demonstrations by Aline Robertson and Alan Isbister] |
|||||||||||||||||||||||||
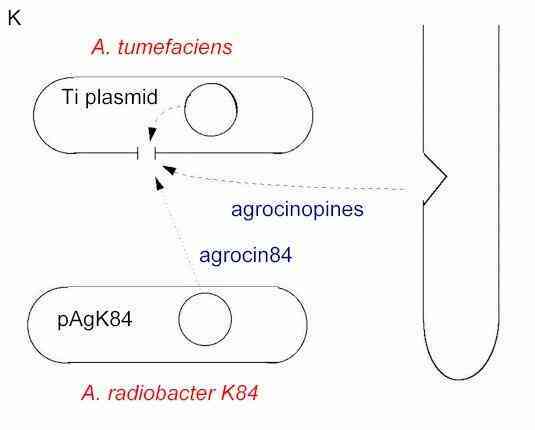
The selective toxicity of agrocin 84 for nopaline producing strains is explained by the fact that these strains also cause the plant to produce agrocinopines, and the Ti plasmid codes for a specific agrocinopine permease, for uptake of these nutrients (Figure K). Agrocin 84 is taken up through this permease (which recognises the sugar group a in Fig. I) and, being a nucleotide analogue, blocks DNA synthesis in the pathogen.
Figure K. Mode of action of agrocin 84. Pathogenic strains of A. tumefaciens with a Ti plasmid that codes for nopaline production also cause the plant to produce agrocinopines. The plasmid codes for an agrocinopine permease, which is inserted in the bacterial membrane. The inhibitor, agrocin 84, is recognised by this permease, enters the pathogen cells and there it blocks DNA synthesis. |
|||||||||||||||||||||||||
Problems and developments in biocontrol A. radiobacter strain K84 is almost a perfect biocontrol agent - even if we had tried to design a control agent we could hardly have done it better! It contains a plasmid, termed pAgK84, which codes of agrocin 84 production. It also contains another plasmid, pNOC, which codes for nopaline uptake and catabolism. So, in natural situations strain K84 might proliferate at gall sites, stealing the pathogen's dedicated source of nutrients (opines), and also killing the pathogen by producing agrocin 84. Added to these points, and of special importance for commercial biocontrol, strain K84 is a very efficient coloniser of healthy plant roots and of wound sites, giving at least some degree of residual protection after it is applied. This efficient colonisation and persistence on roots is at least partly conferred by the chromosomal genes, because many studies have shown that transfer of the agrocin plasmid (pAgK84) into other A. radiobacter strains does not make them as efficient as strain K84. One potential problem threatens the continued success of this biocontrol system, because the agrocin plasmid could, theoretically, be transferred to other cells, including pathogenic strains (or vice versa). This has been demonstrated in laboratory conditions, and if it happened in nature it would result in pathogenic strains that are resistant to agrocin 84 - all organisms that produce inhibitors must also be resistant to the effects of these inhibitors. The agrocin plasmid is not a conjugative plasmid, but pNOC (also present in the biocontrol strain) is conjugative and it can mobilise the agrocin plasmid during its own transfer. The frequency of transfer is enhanced by the presence of nopaline, as would occur in sites where the pathogen becomes established. In order to avoid this potential breakdown of biocontrol, the transfer (Tra) region which aids the mobilisation of the agrocin plasmid has been deleted by genetic engineering, to produce a Tra- (transfer deleted) mutant strain of K84 termed A radiobacter K1026. This genetically engineered strain is now used instead of strain K84 in Australia. It has all the benefits and safety of strain K84, with added stability for biocontrol. The detailed molecular genetical methods used to construct this strain are described by MH Ryder & DA Jones (1991) Australian Journal of Plant Physiology 18, 571-579. Strain K1026 was the first genetically engineered microorganism released for widespread use in the environment. It is entirely safe for humans and other animals (it does not grow at 37oC) and plants, and in all respects except for the deletion of part of its genome it is identical to naturally occurring strains. |
|||||||||||||||||||||||||
A.
tumefaciens has been used extensively for genetic
engineering of plants. This is achieved by engineering
selected genes into the T-DNA of the bacterial plasmid in
laboratory conditions so that they become integrated into
the plant chromosomes when the T-DNA is transferred.
However, several alternative methods also are used for
genetic engineering of plants, including the use of small
DNA-coated microprojectiles which are fired into plant
cells. A few of the commercial applications of these
technologies are shown below.
The transgenic tomatoes do not express the gene for polygalacturonase, an enzyme that degrades pectin, leading to softening of the fruit tissues. As a result, the tomatoes can be left on the plant for longer to accumulate flavour components and they also give a better consistency of tomato pastes. Several crop plants have been engineered to express the insecticidal toxin gene of Bacillus thuringiensis (see Profile on Bacillus thuringiensis), so that insects attempting to eat these plants are killed. This is highly successful, but has the potential disadvantage that continuous exposure of insects to the toxin will select for the development of toxin resistance. Cropping strategies must be devised to "manage" this. Several crops also have been engineered for resistance to herbicides such as glyphosate, so that the herbicide can be used for weed control without damaging the crop. The best-known examples are the "Roundup Ready" crop plants marketed by Monsanto. Other transgenic strategies being explored or commercialised include:
|
|||||||||||||||||||||||||
However, the only essential parts of the T-DNA are its two small (25 base pair) border repeats, at least one of which is needed for plant transformation. So the T-DNA is engineered to remove the genes coding for plant hormones, and a length of DNA is inserted that contains a selectable marker (e.g. an antibiotic-resistance gene; usually kanamycin resistance). This length of DNA must also contain a restriction site - a site with a specific sequence of nucleotides where a restriction enzyme will cut the DNA. For example, the restriction enzyme BamH1 cuts DNA wherever the nucleotide sequence GGATCC occurs (with CCTAGG on the complementary DNA strand). It leaves overlap sites, termed "sticky ends", so that any other piece of DNA cut with the same enzyme can be inserted into this site. The transformation of plants requires:
However, the T-DNA does not need to be on the same plasmid as the Vir genes, so it is often convenient to construct a smaller, self-replicating plasmid containing the T-DNA and to place this in Agrobacterium cells with a "disarmed" Ti plasmid (a binary vector system). Plant transformation can be achieved by incubating Agrobacterium with plant protoplasts. Then the bacterium is killed with an antibiotic, the protoplasts are allowed to regenerate walls and form a tissue culture, the non-transformed cells are killed by addition of kanamycin, and the remaining cells (which survive kanamycin treatment because they have the resistance gene) are used to regenerating plants from tissue culture. Alternatively, Agrobacterium can be added to sterile leaf disks in liquid medium, then hormones are used to induce rooting from the leaf disks and thereby regenerate plantlets. A third method can be used for some plants such as Arabidopsis - the bacterium or even "naked" DNA can be infused through the seed coat to cause transformation. |
|||||||||||||||||||||||||
Further reading Biology of Agrobacterium, and infection behaviour:
Biological control:
Plant transformation:
Crops genetically engineered with Bacillus thuringiensis insecticidal toxin:
Websites (none of these is on this server) Genetic Engineering News: a major source of information on genetic engineering in agriculture and in drug development, etc. Excellent site
from the United States Department of Agriculture
giving many links to Plant Genetic Engineering, including
the use of a natural insecticidal gene from Bacillus
thuringiensis
(Bt): A site from Gene Exchange - "Union of Concerned Scientists" in the USA. |
|||||||||||||||||||||||||

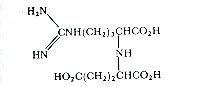 Nopaline
Nopaline