Zoospores of the Oomycota
Structure and ultrastructure
The zoospore body (soma)
of the oomycota is typically kidney-shaped, with a
ventral groove (Figure B). Two flagella emerge about
one-third of the distance along this groove. The
posteriorly directed flagellum is long and smooth - a
"whiplash-type" flagellum. The anterior
flagellum is a shorter "tinsel-type" which
bears tripartite hairs (mastigonemes) along its length.
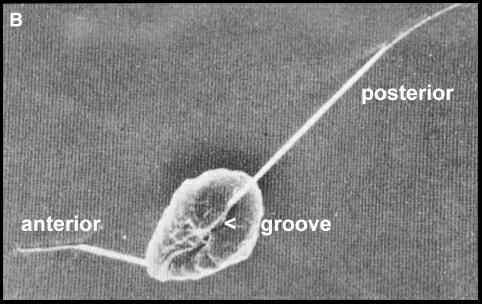
Figure B. Scanning electron micrograph of a
zoospore of Phytophthora [Image supplied by MS
Fuller]
|
When viewed by
transmission electron microscopy (Figure C) these
zoospores show a distinct zonation of organelles. Close
to the ventral groove is the nucleus, which extends as a
beak towards the point where the flagella are inserted.
In a different plane of focus (not shown) this region
also contains a conspicuous water-expulsion vacuole - an
osmoregulatory apparatus. The zoospore also contains many
mitochondria, and vacuoles with striated contents - the
"fingerprint vacuoles". Just beneath the cell
surface are three types of peripheral vesicle - the large
peripheral vesicles, small dorsal vesicles, and small
ventral vesicles. The zoospore is surrounded by a cell
membrane, beneath which is a series of plate-like
membranes - the peripheral cisternae. The functions of
all these peripheral structures are discussed later in
relation to the encystment process. Only a small part of each flagellum
is seen in Figure C. The flagella have a central core
termed the axoneme, composed of microtubules and
associated proteins. Over most of its length, the axoneme
is surrounded by a membrane which is continuous with the
cell membrane. The mastigonemes of the tinsel flagellum
are glycoprotein extensions which project through the
flagellar membrane.
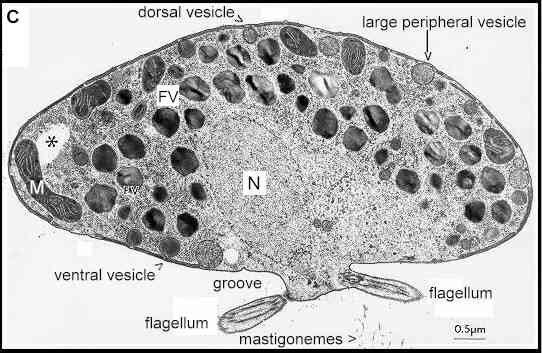
Figure C. Transmission electron micrograph of
a Phytophthora zoospore (about 15 micrometres
diameter) prepared by freeze-substitution and sectioned
through the region where the nucleus (N) extends towards
the flagellar basal apparatus. The spores contain several
mitochondria (M) and fingerprint vacuoles (FV). Three
types of peripheral vesicle are shown, and flattened
peripheral cisternae lie beneath most of the zoospore
plasma membrane. The regions marked with an asterisk are
thought to be where lipids were removed by solvents
during fixation. [Image supplied by MS Fuller; from Cho
& Fuller 1989]
|
Zoospore
swimmingZoospores
can swim for many hours, at 150 micrometres or more per
second, so in theory they could disperse several metres
from their point of release by their own activities. But
in practice this seldom happens because they change
direction frequently when they meet obstacles or by
spontaneous, random turns. They are best suited for local
dispersal but can be carried longer distances in moving
water. In addition, some plant-pathogenic species (e.g. Phytophthora
infestans, which causes potato blight) have
wind-dispersed sporangia and these release zoospores when
they land on a host surface.
The swimming mechanism is
only partly understood. The flagellate bacteria have a
rotor which causes the flagella to rotate, and
spontaneous turns are achieved by a reversal of the rotor
direction. In zoospores there is no flagellar rotor.
Instead, the flagella beat in a sine wave which moves
from the base to tip of each flagellum, and the zoospore
swims in a straight helix, rotating about its axis (see
"control" in Figure E). Hydrodynamic studies
suggest that the posterior flagellum serves mainly as a
rudder, while the anterior flagellum contributes up to
90% of the swimming speed because the mastigonemes act
like oars, converting the forwards-directed sine wave to
a backwards thrust [Reviewed by Carlile, 1983].
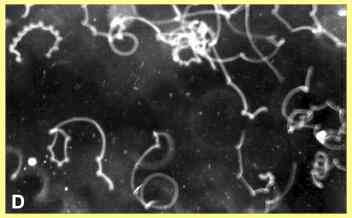
The corkscrew-like pattern
of zoospore swimming, interspersed with random changes of
direction, is shown in Figure D where the tracks of Pythium
zoospores were photographed with a 5-second exposure,
using darkfield microscopy. However, in this case many of
the abrupt turns were caused when zoospores hit the
surface of a glass coverslip.
The
regulation of swimming pattern is strongly influenced by
calcium, which regulates the behaviour of flagella or
cilia in other eukaryotic cells (see Bloodgood, 1989).
For example (Figure E), when external calcium is removed
by a chelator (EGTA) then the zoospores swim in almost
straight lines. They swim in circles when treated with
substances (e.g. lanthanum or verapamil) that block
calcium channels in the cell membrane. They swim in a
skidding fashion when treated with compounds (e.g.
trifluoperazine or dibucaine) that interfere with the
calcium-binding protein calmodulin. And they swim in an
erratic, jerky manner when treated with an ionophore
(which equilibrates the calcium concentration across the
cell membrane) or with amiloride (which disrupts ion
exchange across the membrane).
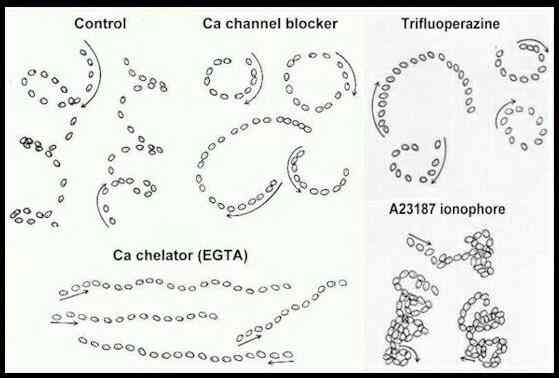
Figure E. Zoospore tracks of Pythium
aphanidermatum treated with various
calcium-interfering substances (Control = no treatment).
Each track shows the position of a single zoospore at
intervals of 0.1 sec (control, channel blocker), 0.2 sec
(EGTA, ionophore) or 0.3 sec (trifluoperazine) traced
from a video screen; arrows show the initial direction of
movement. [Donaldson & Deacon, 1993a].
It is still unclear
exactly how these substances exert their effects on
motility, but two general points are of interest. First, zoospores treated with any of these
substances do not show the normal, periodic changes of
direction and also cannot respond to attractants. So the
normal swimming pattern is essential for the role of
zoospores in site-selection, discussed below. Second,
these findings illustrate one of the many unifying themes
of biology, because calcium plays a central role in all
eukaryotic organisms. In fact, some of these substances
that affect zoospores by interfering with
calcium-mediated processes are used as pharmacological
agents to regulate heartbeat, kidney function or
psychological disturbances. A
nice demonstration of this was reported recently by NASA
scientists, who tested the effects of various substances
on the web-building activities of spiders. This serious
piece of research showed that caffeine was one of the
most disruptive agents. Caffeine exerts its effects by
depleting the calcium levels in intracellular stores.
"Fortean Times" (The
Journal of Strange Phenomena) picked up on this with a
wonderful joke (or was it an advert?):
Based on a page in "Fortean
Times", Number 84
Caffeine strongly
disrupts normal zoospore function, and ethanol is a
strong chemoattractant of some zoospores!
|
| The zoospore homing sequence Having laid the
foundations of zoospore structure and function, we now
consider the homing sequence leading to infection of a
host from a zoospore. The following diagram gives a
composite view of this sequence, starting from a
sporangium, such as one of the wind-dispersed sporangia
of Phytophthora infestans (the cause of potato
blight). However, it should be noted that this composite
view has been built up from studies on various Phytophthora,
Pythium and Aphanomyces species. Not
all of these stages may apply to any one fungus.
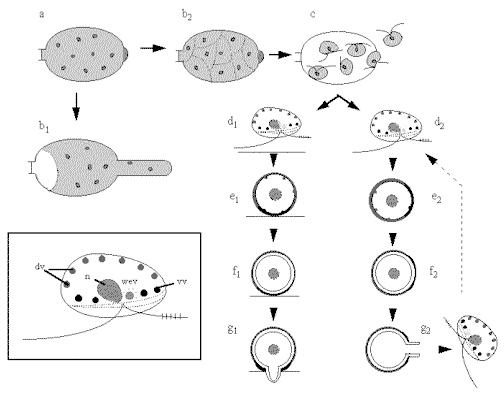
Stage a (top
left) in this diagram shows a sporangium of a Phytophthora
infestans. In this fungus the sporangium can
germinate in two ways: by hyphal outgrowth (b1)
or by undergoing protoplasmic cleavage (b2)
to release zoospores (c) by dissolution of the
sporangial papillum. Zoospores (inset at bottom
left) have an anterior tinsel flagellum and a posterior
whiplash flagellum, inserted in a ventral groove close to
the position of the nucleus (n) and water-expulsion
vacuole (wev). Small dorsal vesicles (dv) and small
ventral vesicles (vv) lie just beneath the plasma
membrane. The sequence d1 to
g1 shows the events
when a zoospore is induced to encyst by a host surface
component. The spore typically orientates, settles and
adheres with the future, pre-determined germination site
against the host. (e1). Cyst
coat glycoprotein is then released by exocytosis of the
small dorsal vesicles, and proteinaceous adhesive is
released from the ventral vesicles. Over the next few
minutes a microfibrillar cyst wall is synthesised beneath
the cyst coat (f1). Then after
20-30 min the cyst germinates by a hyphal outgrowth
towards the host (g1); the hypha
either infects directly or (not shown) produces a swollen
appressorium before infection. An alternative sequence (d2-g2)
occurs if a zoospores does not find a host. It encysts
eventually and, after some hours, typically releases a
further zoospore.
|
The germination of sporangia is discussed later (see Germination
of sporangia) but in the
following sections we focus on the zoospores and consider
four of the most important stages leading to infection of
a host (Figure F). 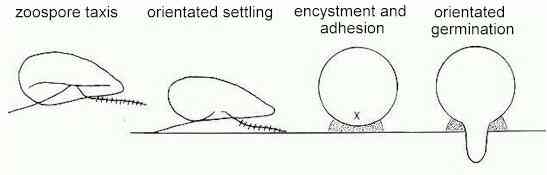
Figure F.
- 1. accumulation of zoospores
at specific sites by sensing gradients of
attractants or repellents (zoospore taxis);
- 2. settling and orientation on
the host surface, perhaps by recognition of
specific host surface components;
- 3. adhesion and encystment,
involving the release of adhesins and production
of a cyst wall;
- 4. germination with a fixed
orientation, from an apparently predetermined
point.
These four stages are
remarkable for many reasons:
they occur so
rapidly that they can lead to infection from an
originally motile spore within 30-40 minutes;
during his
short time the cells undergo major changes of
organisation - the originally wall-less, motile
cell is transformed into a walled cyst which then
gives rise to a new hyphal apex (see apical growth);
the cells can
respond to many exogenous cues, including those
that change the direction or speed of swimming,
others that trigger encystment and yet others
that can trigger cyst germination;
throughout
this time the cells do not take up external
organic nutrients - this occurs only after the
cyst has germinated and the germ-tube has grown
to some length.
Thus, we can think of
zoospores as being largely pre-programmed cells, which
require only the appropriate signals to progress through
a complex sequence of events leading to infection.
|
Zoospore
taxisThe
term taxis can be used generally to
describe a change in swimming orientation of a motile
cell in response to an external stimulus. The zoospores
of various fungi have been shown to respond to chemical
gradients (chemotaxis), oxygen (aerotaxis),
electrical or ionic fields (galvanotaxis,
electrotaxis) or light (phototaxis).
Some also can swim against a water current (rheotaxis)
and some swim upwards in suspension (negative
geotaxis) but probably not by sensing gravity as
such. In addition to all these factors, when zoospore
suspensions are sufficiently dense then the cells can
autoaggregate - a phenomenon termed adelphotaxis
and perhaps caused by the release of an attractant
substance.
One or more of these
factors can cause zoospores to accumulate at specific
sites. For example, the zoospores of plant pathogenic
fungi commonly accumulate at wound sites, or near the
stomata of leaves, or near the root tips (Figure G).
Similarly, the zoosporic parasites of small animals often
accumulate at the body orifices (see Catenaria).
Zoospore taxis can also
serve to bring zoospores to sites where they will be
destroyed by toxins. A natural example of this is seen in
the responses of zoospores to oat roots: see zoospores and saponins.

Figure G. Videotape images (shown as
negatives) of Pythium zoospores accumulating on
wheat roots: at 1 min 43 sec (left) and 8 min 25 sec
(right) after roots were immersed in zoospore suspension.
The left image shows a root tip surrounded by a ball of
root tip mucilage containing shed root cap cells (rc). A
mass of zoospores (z) has accumulated over the surface of
the root tip mucilage. The right image shows a region
just behind the tip of a root coated with two layers of
alginate gel. Zoospores (z) have accumulated locally on
the alginate in the zone where root hairs start to
emerge. [From Jones et al., 1991]
|
Capillary model systems can be used to investigate the
individual attractants of zoospores (Figure H) and thus
give clues to the factors involved in host-location.
Typically, the zoospores of root-infecting Pythium
and Phytophthora species are attracted strongly
to a few individual amino acids (e.g. aspartic or
glutamic acid), sugars (e.g. glucose) or volatile
compounds (e.g. ethanol, aldehydes), all of which are
common components of root exudates. But the spores
respond better to mixtures than to individual compounds.
In addition, a few examples of host-specific attractants
have been reported from capillary studies. For example, Phytophthora
sojae, a host-specialised pathogen of soybean roots,
is strongly attracted to the soybean flavonoids daidzein
and genistein, and Aphanomyces cochlioides (from
spinach) is strongly attracted to the host flavonoid,
cochliophilin A. These reports are interesting because
they parallel the behaviour of host-specific Rhizobium
species, which also are attracted to the flavonoids of
their hosts and can be repelled by non-host flavonoids.
It is assumed that the
responses to different attractants are mediated by
chemoreceptors in the zoospore or flagellar membrane. But
no receptor has yet been purified and characterised from
a zoosporic fungus.
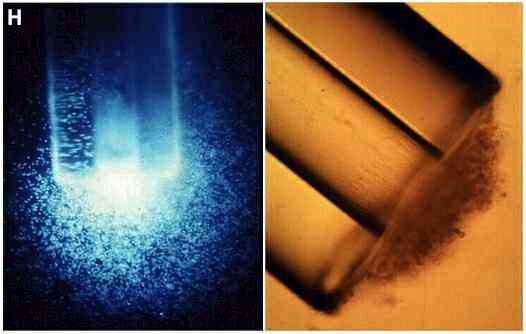
Figure H. Left: zoospores of Phytophthora
palmivora accumulating at the mouth of a capillary
filled with a fluorescently tagged amino acid
(dansyl-asparagine) [supplied
by MJ Carlile, from JN Cameron & MJ Carlile, unpubl.]. Right: zoospores of Pythium
aphanidermatum accumulated and encysted at the mouth
of a capillary containing malt-extract agar. Germ-tubes
have grown from the cysts into the agar in the capillary.
|
Zoospore
encystmentZoospores
can be induced to encyst by artificial agents such as
high concentrations of chemicals or mechanical agitation
in laboratory conditions. But in nature they probably
respond to recognition of a host surface component. For
example, the zoospores of many Pythium and Phytophthora
species are induced to encyst by pectin or by root
surface mucilage (which often has a similar composition
to pectin). There may also be a degree of specific
recognition. For example (see Table 2) three species of Pythium
responded differently to a range of plant-derived
polymers in laboratory assays, and the Pythium
species that characteristically infect the roots of
grasses and cereals show only a weak ability to encyst on
non-grass roots (Table 3).
Table 2. Percentage of zoospores induced
to encyst by addition of different polymers;
significant differences from the controls (no
treatment) are shown in bold. Some treatments are
shown only as "yes" (encystment
induced) or "no" (no effect).
|
| Polymer |
Fungus |
| |
P.aphanidermatum |
P.catenulatum |
P.dissotocum |
| Control (none) |
10 |
12 |
25 |
| Arabinoxylan |
61 |
18 |
26 |
| Methylglucuronoxylan |
10 |
54 |
23 |
| Xyloglucan |
10 |
17 |
73 |
| Fucoidan |
7 |
54 |
25 |
| Mixed linkage glucan |
11 |
45 |
39 |
| Gum arabic |
37 |
69 |
65 |
| |
| Alginate |
Yes |
Yes |
Yes |
| Cellulose |
No |
Yes |
Yes |
| Chitin
(crab shell) |
Yes |
No |
Yes |
Data
from Donaldson & Deacon (1993b); RT Mitchell &
Deacon (unpubl.)
|
| Table 3. Numbers of zoospores that
encysted on seedling roots of wild grasses or
wild dicotyledonous plants in replicated
laboratory tests |
| |
| |
Pythium
graminicola |
Pythium
aphanidermatum |
| |
Grass (A) |
Dicot (B) |
A/B |
Grass (A) |
Dicot (B) |
A/B |
| Expt 1 |
279 |
28 |
10.0 |
248 |
243 |
1.1 |
| Expt 2 |
112 |
44 |
2.6 |
94 |
103 |
0.9 |
| Mean of 9
experiments |
3.6 |
|
1.0 |
Seedlings of wild grasses and dicotyledonous
plants were collected from field sites and
immersed in zoospore suspensions of either P.
aphanidermatum (which has a
characteristically broad host range) or P.
graminicola (which characteristically
infects Gramineae). In each of 9 experiments with
replications within the experiments, similar
numbers of P.aphanidermatum zoospores
encysted on grass and dicot roots, whereas P.
graminicola encysted mainly on grass roots.
Presumably P. graminicola zoospores
recognise components of the root surface mucilage
of Gramineae, which is substantially different
from the root mucilage of other plants. [Data
from Mitchell & Deacon, 1986a] |
|
Several events occur in quick succession during zoospore
encystment, suggesting that the cells are pre-programmed
to respond to an initial encystment signal.
1. The flagella are
shed, the zoospore rounds off, and the nucleus
migrates to a central position.
2. Some of the
peripheral vesicles (see Figure C) fuse with the cell membrane
to release their contents onto the cell surface.
These contents include a cyst coat glycoprotein from
the small dorsal vesicles and an adhesive protein or
glycoprotein from the small ventral vesicles.
3. The flat peripheral
cisternae start to bud vesicles which will deposit a
cyst wall underneath the cyst coat.
4. The production of
the cyst wall is usually completed in about 5-6
minutes. Then the water-expulsion vacuole disappears,
and a germ-tube emerges about 20-30 minutes later, to
produce either a hypha or an appressorium
(pre-infection swelling).
The disruption of any of these
events could potentially lead to succesful control of
disease caused by zoosporic fungi. An interesting example
of this is the role of oat roots in disrupting the normal
behaviour of zoospores - see Zoospores and saponins
|
Zoospore alignment and orientation
of germinationWhen zoospores settle on a host surface, the
cysts almost invariably germinate towards the host
(Figure I). This raises the possibility that zoospores
might pre-align during encystment. Several lines of
evidence support this idea and also suggest that the site
of germination is predetermined. In other words, a
zoospore settles and encysts on a host with a precise
orientation (docking) so that the emerging hypha can
rapidly penetrate the host.
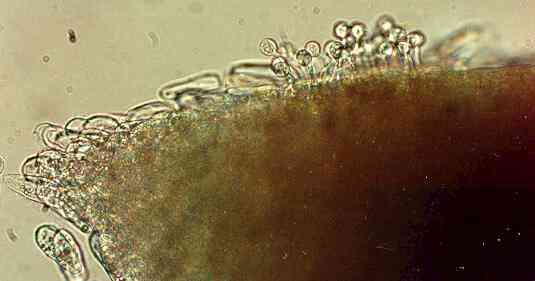
Figure I. Zoospore cysts of Pythium
aphanidermatum germinating towards a wheat root
after the zoospores settled and encysted just behind the
root tip.
|
The first evidence for
pre-alignment came from simple laboratory experiments with Pythium zoospores,
shown diagrammatically in Figure J. Motile spores were
placed in a chamber with a block of nutrient-rich agar
(malt-peptone agar) at one end and a block of
nutrient-free water agar at the other end. Most of the
zoospores swam towards the nutrient-rich agar, then
encysted and germinated towards the agar block. Fewer
zoospores settled and encysted near the water agar, and
these cysts germinated with random orientation.
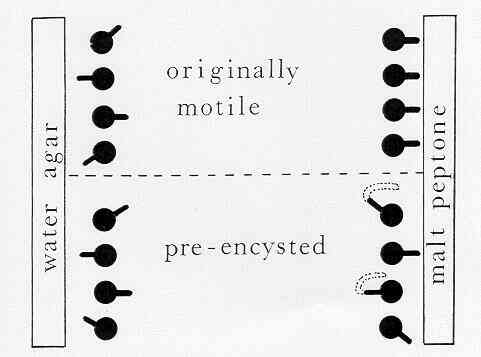
Figure J. Simple experiment to show that zoospores
of Pythium have a fixed (predetermined) point of
germination. (see Mitchell & Deacon, 1986b)
These findings could be
explained in two ways:
- either the
zoospore has a fixed (predetermined) site of
germination which is located next to the
attractant source (e.g. host) by pre-alignment of
the zoospore when it encysts
- or the
zoospore cyst has the potential to germinate from
any point, and the actual site of germination is
influenced by the attractant source (e.g. host).
To distinguish between
these possibilities, zoospores were encysted artificially
(by agitation) then placed in a chamber. The pre-encysted
cells always germinated with random
orientation, even when they were positioned next to the
nutrient-rich agar. So they seem to have a fixed site of
germination which cannot be influenced by external
factors such as nutrients. Nevertheless, the germ-tubes
can change direction after they have
emerged from a fixed position, and then grow towards a
nutrient source (see hyphal tropisms).
|
| Further
understanding of these events has come from the use
monoclonal antibodies (MAbs) raised against components of
zoospores of Phytophthora cinnamomi (see
Hardham, 1995). Some of these MAbs are specific
to the glycoprotein contents of dorsal vesicles in the
zoospores (see Figure C). By MAb-tagging, this glycoprotein was
shown to be released by exocytosis during encystment and
it forms the cyst coat. Other MAbs are specific to the
protein or glycoprotein contents of ventral vesicles,
which line the shoulders of the zoospore ventral groove.
This material also was released during encystment, and
was shown to be deposited next to a host surface, where
it is thought to function as an adhesive. Thus, the
zoospores must pre-align during encystment, with the
ventral groove next to a host surface. This raises the possibility that
the flagella might be involved in recognising host
surface components, and again evidence has come from the
use of MAbs. Of all the antibodies raised against
zoospores of P. cinnamomi, only one caused rapid
encystment when added to motile spores. This MAb binds
specifically to the surface of both
flagella, and it also induced rapid encystment of other Phytophthora
and Pythium species. In contrast, MAbs that bind
to the whole zoospore surface or to components of only
the anterior flagellum did not cause rapid encystment.
|
Role of calcium in cyst germinationWe noted earlier that calcium has an
important role in zoospore motility. It also is required
for cyst adhesion and subsequent cyst germination. This
is not surprising because calcium is an important second
messenger in all eukaryotic cells: it is intimately
involved in the signal transduction pathways
that link the perception of an external stimulus to a
cellular response. For example, the binding of a ligand
to the external face of a membrane receptor needs to be
translated into a cellular response, such as the
activation of a gene or other change of cell behaviour.
Calcium ions are one of the links in this chain. The
binding of the receptor might open a calcium-specific
membrane channel, allowing a localised influx of Ca2+.
Any change in the intracellular free Ca2+
content can then have numerous effects, via interaction
with cytoskeletal proteins or with calcium-binding
proteins such as calmodulin.
All the substances that interfere
with calcium-mediated processes can disrupt cyst
germination when applied to pre-encysted cells. They
include calcium channel-blockers, calcium ionophores,
calcium chelators, calmodulin inhibitors, and compounds
such as caffeine that deplete the calcium levels in
intracellular stores, or other compounds (e.g. TMB-8)
which block the release of calcium from intracellular
stores. Equally important, zoospore cysts have an
absolute requirement for external calcium, because the
removal of this with calcium chelators inhibits
germination.
These findings have spurred
attempts to measure calcium changes in encysting
zoospores. One approach to this is the use of
calcium-sensing dyes, which change their fluorescent
properties when they bind to calcium. For example, some
of the "ratiometric dyes" change the excitation
wavelength at which they fluoresce when exposed to
calcium, so the ratio of fluorescence at different
excitation wavelengths can be used to estimate
intracellular free calcium levels in a cell. Figure K
shows examples of living zoospores which absorbed these
dyes and were observed by confocal microscopy. The
calcium levels in different parts of the cells are then
translated into false colour images. But in these
examples the dyes were rapidly sequestered into cellular
organelles - a common problem in fungi.
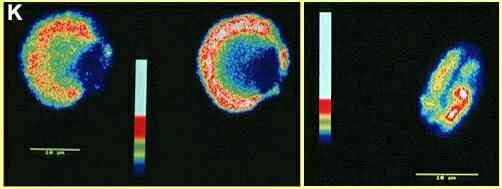
Figure K. False colour
images obtained by confocal microscopy of Phytophthora
zoospores treated with the calcium-sensing dyes fluo-3
(left) and calcium green (right). The left-hand image
shows a recently encysted zoospore in two optical
sections (towards the top of the cell - left; in near
median plane of focus - right). The blue region, of
lowest free calcium concentration, corresponds to the
position of the nucleus and water-expulsion vacuole. The
higher calcium concentrations (red or white) seem to be
in a zone where many fingerprint vacuoles and
mitochondria occur, suggesting that the dye was
sequestered in these organelles. The right-hand image
shows an optical section through a motile zoospore. The
blue zone along the centre of the cell represents the
ventral groove. [Adrian Warburton & JW Deacon,
unpubl.]
|
The problem of dye sequestration in
organelles can be overcome to some degree by using
cell-impermeable (free acid) forms of the dyes, which
remain outside of the cells so that fluorimetry can be
used to measure changes in the calcium in the bathing
medium of a population of zoospores. The dyes then
measure the fluxes of calcium ions into and out of the
cells during the stages of zoospore encystment. Recent
studies of this sort (Figure L) show that the external
calcium concentration drops markedly when zoospores are
induced to encyst by agitation, indicating that the
spores take up a large amount of calcium at this stage.
Then they release calcium progressively during the next
20-30 minutes, representing an enormous net efflux of the
cellular calcium reserves. When the experiment is
repeated in the presence of a calcium channel blocker
(lanthanum or verapamil) there is no net influx or efflux
of calcium (Figure M), and the zoospores do not encyst.
If TMB-8 is used instead of a channel blocker, the spores
show an initial net influx of calcium, but no subsequent
efflux (Figure N). These cells encysted but showed only
low germination. [In mammalian systems, TMB-8 is known to
block the release of calcium from intracellular calcium
stores]
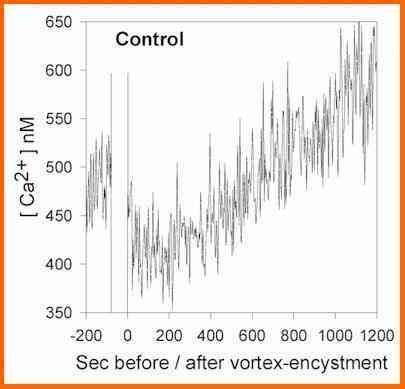
Figure L. Estimates of the free calcium concentration in
the medium surrounding a population of Phytophthora
zoospores, as measured by fluoriemtry of the
calcium-sensing dye fura-2. Calcium levels in the medium
were measured for an initial 200 seconds, then the cells
were vortexed to induce encystment (70 seconds break in
the diagram) before the recordings were continued. [A
Warburton & JW Deacon, 1998]
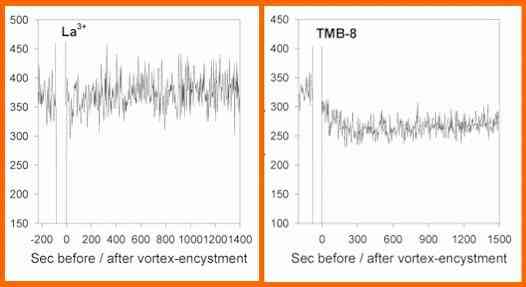
Figure M. Estimates of the free calcium concentration in
the medium surrounding a population of Phytophthora
zoospores. Details as in Figure L except that lanthanum
or TMB-8 was added immediately before the zoospores were
vortex-encysted. [A Warburton & JW Deacon, 1998]
Studies such as these
could form the basis for novel approaches to control
zoosporic plant pathogens. The manipulation of external
calcium levels can have profound effects on several
stages of the zoospore homing sequence, of possible
practical benefit in glasshouse irrigation systems (von
Broembsen & Deacon, 1997).
|
| Germination of
sporangia Recent work has
extended some of the above findings to the airborne
sporangia of Phytophthora infestans, which
causes potato late blight and was responsible for the
Great Irish Potato Famine in the 1840s. It is still a
significant pathogen of potato crops. In laboratory
conditions these sporangia will release zoospores when
incubated in water at 12oC, or germinate by
hyphal outgrowth at 20oC. In either case, the
germination of sporangia is suppressed by a wide range of
treatments that would be expected to combine with any
external calcium ions (e.g. calcium chelators such as
EGTA or BAPTA, or even phosphate or pectin). Moreover, as
shown in the diagram below, these treatments lead rapidly
to death of the sporangia. This raises the prospect that
we might be able to use mild, environmentally safe
chemicals to control potato blight. But the effects have
yet to be tested in realistic field conditions.
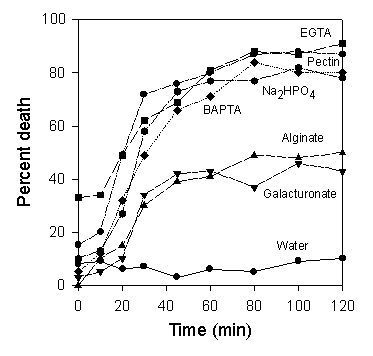
Death of Phytophthora
infestans sporangia when incubated in the presence
of 0.1% or 5 mM concentrations of various chemicals at 12oC
in laboratory conditions. From Hill et al., 1998.
|
| Further reading: Reviews
RA Bloodgood (1991)
Transmembrane signalling in cilia and flagella. Protoplasma
164, 12-22.
MJ Carlile (1983)
Motility, taxis and tropism in Phytophthora.
In Phytophthora: Its Biology, Taxonomy, Ecology
and Pathology (ed. DC Erwin, S Bartnicki-Garcia
& PH Tsao) pp. 95-107. American Phytopathological
Society, St Paul.
JW Deacon & SP
Donaldson (1993) Molecular recognition in the homing
responses of zoosporic fungi, with special reference
to Pythium and Phytophthora. Mycological
Research 97, 1153-1171.
AR Hardham (1995) Polarity of
vesicle distribution in oomycete zoospores:
development of polarity and importance for infection.
Canadian Journal of Botany 73
(Supplement) S400-S407.
Research articles (hyperlinks go to Abstracts
of recent papers)
CW Cho & MS Fuller
(1989) Ultrastructural organization of
freeze-substituted zoospores of Phytophthora
palmivora. Canadian Journal of Botany 67,
1493-1499.
SP Donaldson & JW
Deacon (1993a) Changes in motility of Pythium
zoospores induced by calcium and calcium-modulating
drugs. Mycological Research 97,
877-883.
SP Donaldson & JW
Deacon (1993b) Differential encystment of zoospores
of Pythium species by saccharides in
relation to establishment on roots. Physiological
and Molecular Plant Pathology 42, 177-184.
SW Jones, SP Donaldson
& JW Deacon (1991) Behaviour of zoospores and
zoospore cysts in relation to root infection by Pythium
aphanidermatum. New Phytologist 117,
289-301.
RT Mitchell & JW
Deacon (1986a) Differential (host-specific)
accumulation of zoospores of Pythium on
roots of graminaceous and non-graminaceous plants. New
Phytologist 102, 113-122.
RT Mitchell & JW
Deacon (1986b) Chemotropism of germ-tubes from
zoospore cysts of Pythium spp. Transactions
of the British Mycological Society 86,
233-237.
SL von Broembsen &
JW Deacon (1997) Calcium interference with zoospore
biology and infectivity of Phytophthora
parasitica in nutrient irrigation solutions. Phytopathology
87, 522-528.
A Warburton & JW
Deacon (1998) Transmembrane Ca2+ fluxes
associated with zoospore encystment and cyst
germination by the phytopathogen Phytophthora
parasitica. Fungal Genetics and Biology 25,
54-62.
AE Hill, DE Grayson
& JW Deacon (1998) Suppressed germination and
early death of Phytophthora infestans
sporangia caused by pectin, inorganic phosphate, ion
chelators and calcium-modulating treatments. European
Journal of Plant Pathology 104,
367-376.
Websites
Plasmodiophorid Home Page
Zoosporic Fungi Online
GO
TO FULL LIST OF PROFILES?
|