This Profile follows
from the introduction given in Fungal
Zoospores I. It deals with
the zoospores of two further groups of organisms - the
chytridiomycota and plasmodiophorids, and their roles as
vectors of some important plant
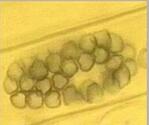
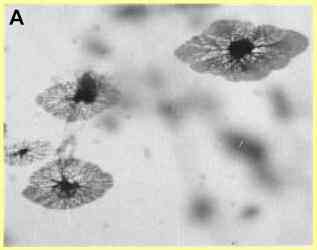
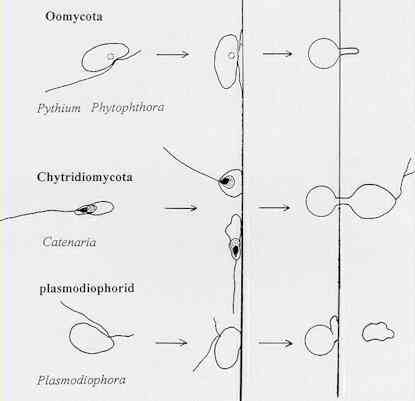
viruses. 1. Chytridiomycota The chytridiomycota are the only major group of true (chitin-walled) fungi that produce zoospores. These fungi are very common as saprotrophs, facultative parasites and obligate parasites in moist soil and freshwater habitats. They depend on their zoospores for dispersal and site-selection. In fact, this is how the chytridiomycota can be detected - by placing baits such as plant seedlings, pollen grains, insect exoskeletons etc. in water or wet soil, because the zoospores encyst on these materials and then give rise to microscopic colonies (Figure A).
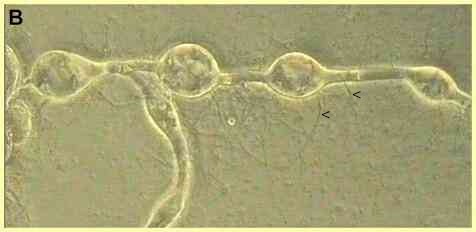
The saprotrophic species include several that degrade polymers. For example, Rhizophlyctis (Karlingia) rosea colonises and degrades cellulose baits in soil, and Chytridium confervae degrades chitin. A specialised group of chytrids grow in the rumen of herbivorous animals, where they degrade cellulose and thus play a primary role in the complex microbial ecology of the rumen. These fungi, such as Neocallimastix frontalis, are unusual because they are obligately anaerobic. They ferment sugars to generate a mixture of formate, acetate, lactate, ethanol, CO2 and H2. The facultative parasitic species include Catenaria anguillulae, a parasite of nematodes, fluke eggs and several other small organisms (see Figure B). Some other facultative parasites attack the resting spores of plant-pathogenic fungi or mycorrhizal fungi in soil, and perhaps contribute significantly to natural biocontrol systems.
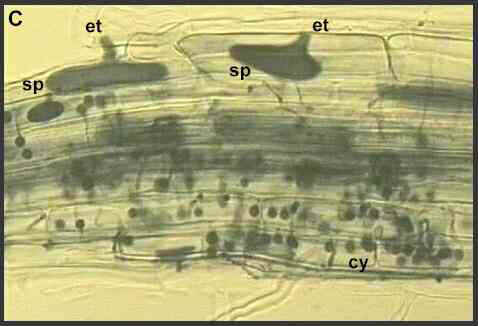
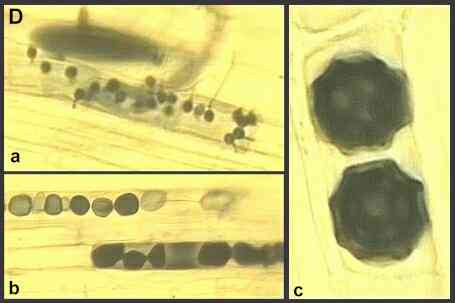
The obligately parasitic species include Olpidium brassicae and other Olpidium species, which are extremely common on roots of wild and crop plants throughout the world. These fungi cannot be grown in culture, away from their hosts. They are symptomless parasites which cause no damage in their own right, but they are important vectors of several soil-borne plant viruses (see later). Stages in the life cycle of these fungi, relevant to their roles as virus vectors, are shown in Figures C and D.
|
|||||||||||||||||||||||||||||
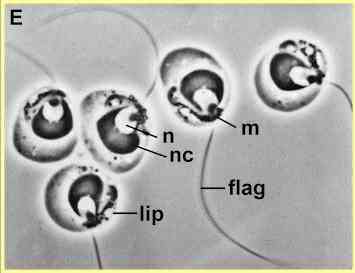
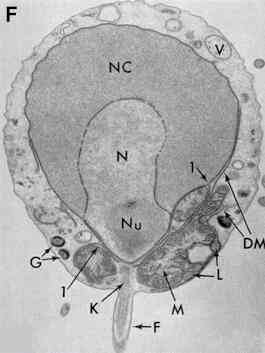
Zoospore ultrastructure
[Figures E and F supplied by MS Fuller, from Reichle & Fuller, 1967] |
|||||||||||||||||||||||||||||
| Zoospore biology The basic biology of chytridiomycota zoospores is similar to that of the oomycota, although there are some differences in detail. Zoospore chemotaxis has been studied for several chytridiomycota, but perhaps the most complete study is for the rumen chytrid Neocallimastix frontalis (Orpin & Bountiff, 1978). In rumen fluid the zoospores showed strong chemotaxis to the awns and other inflorescence parts of barley. In capillary tests on single compounds, chemotaxis was observed towards a range of sugars and sugar derivatives, but not to the common amino acids, purines, pyrimidines and vitamins. By testing the responses to attractants in the presence of high background levels of other compounds (levels that should give saturation binding to receptors) these workers deduced that there are four chemoreceptors on the zoospores:
Of all the single compounds tested, the zoospores showed strongest attraction to sucrose, glucose and fructose - the three sugars detected in largest amounts in barley inflorescence tissues - and the strongest attraction was found to a mixture of these three sugars at the concentrations found in awn and inflorescence tissues. Zoospore settling and encystment has been studied in several chytridiomycota, with some evidence of differential encystment on different materials (Table 1). For example, zoospores of the strongly cellulolytic fungus Rhizophlyctis rosea were induced to encyst after making random contact with pieces of transparent cellulose film or other cellulose sources (cotton threads, etc.) but did not respond to pieces of chitin. Conversely, zoospores of the strongly chitinolytic fungus Chytridium confervae were induced to encyst by chitin but not by cellulose. All possible combinations of responses to these materials were found among the fungi tested (Table 1).
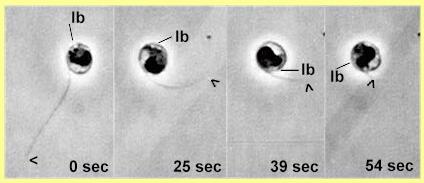
Orientation of encystment in chytridiomycota typically involves a period of amoeboid crawling over a surface (see Catenaria). The anterior sac-like region of these zoospores can send out several pseudopodium-like extensions. Throughout this crawling phase the flagellum is held above the surface, so any cell surface receptors involved in encystment are probably located on the zoospore body, not the flagellum. Finally, in Catenaria the zoospore changes to a rounded shape with the flagellum projecting away from the host surface, and the flagellum is retracted into the zoospore. As shown in Figure G, this retraction is accompanied by rotation of the cell contents. |
|||||||||||||||||||||||||||||
From frame 1 (0 sec) to frame 4 (after 54 sec) the flagellum is retracted progressively into the cell. This is accompanied by rotation of most or all of the cell contents. The point where the flagellum enters the cell remains in the same position, so it seems that the cell itself remains stationary while the flagellum is reeled in. [From Deacon & Saxena, 1997] |
|||||||||||||||||||||||||||||
It seems likely that the zoospores of most groups of organisms have a fixed orientation of encystment and germination, as in the oomycota (Figure H). But in both chytridiomycota and plasmodiophorids (e.g. Plasmodiophora brassicae) the zoospores seem to settle with the flagella projecting away from a host surface, based on limited evidence to date. It is not possible to say whether the actual site of germination is pre-determined in chytridiomycota, because the cellular markers change position during flagellar retraction.
|
|||||||||||||||||||||||||||||