There are many examples of symbiosis in nature, but lichens are unique because they look and behave quite differently from their component organisms. So, lichens are regarded as organisms in their own right and are given generic and species names. However, for taxonomic purposes the names are actually fungal names: lichens are regarded as a special group of fungi - the lichenised fungi. There are an estimated 13,500 to 17,000 species of lichens, extending from the tropics to the polar regions. Some of them grow on the bark of temperate trees or as epiphytes on the leaves of trees in tropical rain forests. Others occupy some of the most inhospitable environments on earth, growing on cooled lava flows and bare rock surfaces, where they help in the process of soil formation, and on desert sands where they help to stabilise the surface and enrich it with nutrients (see Cyanobacteria). Some other types of lichen grow abundantly on tundra soils, providing a vital winter food source for animals (including reindeer and caribou) in arctic and sub-arctic regions.Yet other lichens grow on or in the perennial leaves of some economically important tropical crop plants such as coffee, cacao and rubber, where they are regarded as parasites. All these features make lichens interesting and significant in environmental terms. But lichens also pose challenging scientific problems - how do two or more microorganisms interact at the cellular, genetical and biochemical levels to produce a unique, hybrid organism? |
||||
The types of lichen The "body" of a lichen is termed the thallus, and its general shape enables us to group lichens into four broad categories.
|
||||
 Foliose lichens. (A) Parmelia physodes, growing on the twigs of a shrub. The lobes, about 1 cm diameter, are silvery-grey above, black below. (B) Peltigera polydactyla, growing on soil. The lobes are semi-erect, 1-2 cm diameter, and have brown fungal fruiting bodies (ascocarps) at their tips (see later). In this case the lichen thallus is grey because it has dried, but it rapidly becomes bluish-green when rewetted. |
||||
 Fruticose
and squamulose lichens. (C) Usnea comosa, growing on a wooden
post. This lichen has a branched,
filamentous thallus which hangs down from the point of
attachment. (D) Cladonia pyxidata, growing
on peaty soil. This lichen has a scale-like squamulose
structure (arrowhead) but also produces erect stalked
cups termed podetia. (E) Cladonia
coccifera, similar to C. pyxidata but the rims
of the podetia bear many conspicuous red ascocarps. |
||||
 Crustose lichens. (F) A patchwork of lichens on a rock surface, including several colonies of the green-coloured Rhizocarpon geographicum and the white Lecidia species. (G) Lecanora muralis (also known as Squamaria muralis) growing on a wall. The thallus has a pale green colour with small lobes at the margin but the centre is crustaceous. Also present are many pale brown ascocarps. |
||||
The lichen partnership A few lichen fungi (approximately 20) are members of the fungal group Basidiomycota, but the vast majority are members of the Ascomycota (ascus-forming fungi) and they often produce conspicuous fungal fruiting bodies (ascocarps) - usually disk-shaped structures termed apothecia (e.g. Figures E, G). Almost half of the recorded fungi in the world are ascomycota, and nearly half of these are found only in lichens. Although their spores are dispersed from the fruiting bodies, these fungi do not seem to have an independent role in nature because they are extremely slow-growing and generally lack the enzyme systems for degrading complex polymers. In contrast to the many thousands of lichen fungi, there are only about 100 photosynthetic partners. The most common are single-celled green algae of the genus Trebouxia (Figure H), which are found in many lichens of temperate and arctic/alpine regions, including all species of the common lichen genus Cladonia (see Figures D, E). Trebouxia species seldom grow as free-living cells in nature; instead they seem to be specialised lichen symbionts. Another common photobiont is the filamentous green algal genus Trentepohlia, especially in Mediterranean and tropical regions. This and other algal genera of the tropical lichens can be found growing independently in nature. About 10% of lichens have cyanobacteria (e.g. Nostoc) as the main or only photosynthetic partner. For example, this is true of many Peltigera species (Figure B). However, some lichens that contain green algae can also have cyanobacteria in special wart-like structures on the lichen surface. These structures are termed cephalodia. They are found in about 3-4% of lichen species and their role is probably to exploit the nitrogen-fixing ability of cyanobacteria. The fact that lichens can be formed by more than one type of fungus and more than one type of photosynthetic partner shows us that the lichen symbiosis must have evolved independently on several occasions. |
||||
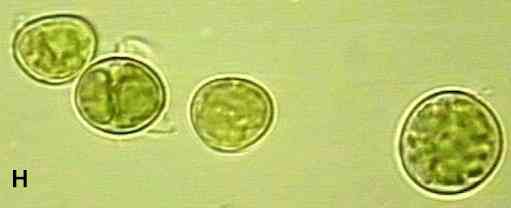 The single-celled green alga, Trebouxia |
||||
The structure of lichens Lichens vary in their degree of structural organisation. At one extreme are some simple, almost casual associations between fungal hyphae and photosynthetic cells, in which there is little structural modification. These simple associations may be quite common but are inconspicuous. The more conspicuous lichens have a well-defined structure, often with distinct zonation of the partners, as shown in Figures I-K, below. Usually, the photosynthetic cells are found in a defined band where they are closely associated with fungal hyphae, for nutrient exchange. Above this band is a cortex consisting of tightly packed fungal cells with a gelatinous matrix between them. Within and below the photosynthetic zone is a medulla of loosely packed hyphae. Often there is a thinner, lower cortex, and several types of lichen attach themselves to a surface by root-like structures termed rhizinae or rhizines (see Fig. K). |
||||
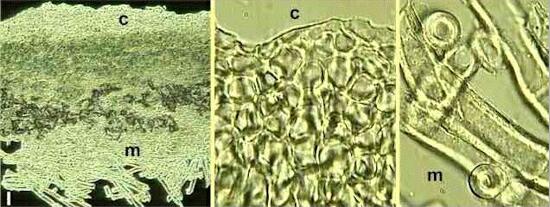 Figure I. Structure of the lichen Peltigera polydactyla. In cross section (left) the lichen thallus is seen to consist of an upper cortex (c) of tightly packed fungal cells resembling a tissue. This is seen at higher magnification in the centre image. Beneath the cortex is a medulla (m) of more typical fungal hyphae (seen at higher magnification in the right-hand image). The photosynthetic cells (a cyanobacterium in this case) are seen as a green-coloured band beneath the cortex. The lichen also has a lower cortex, beneath the medulla, but it is not seen in this section. Note the air pockets in the upper part of the medulla, close to the photosynthetic zone (grey-black regions in the left-hand image). They indicate that the fungal hyphae in this region are hydrophobic. Note also the extremely thick walls of hyphae in the medulla (the right-hand image). These thick-walled, hydrophobic hyphae contribute to drought-tolerance. |
||||
 Figures J, K. Structure of the common foliose lichen Xanthoria parietina, which grows on many types of surface, including concrete, roofs of buildings, and rocks subjected to sea spray. The lichen thallus (J) has foliose lobes at the margin (see top of Figure J) but much of the surface is covered with bright orange apothecia about 3-5 mm diameter (arrowheads). The orange colour of this lichen is due to production of the pigment parietin at the lichen surface. (K) Cross section of one of the marginal lobes, viewed by phase-contrast microscopy. The photosynthetic zone (p) is seen as a distinct band of green algal cells. Above this band is the cortex (c) consisting of densely packed fungal cells . The surface of the cortex is pigmented because the cell walls are impregnated with parietin. The lower part of the thallus consists of a medulla (m) with conspicuous air pockets (a), and a thin lower cortex (lc). Like many foliose lichens, Xanthoria produces rhizinae (r) that penetrate into crevices and help to anchor the lichen to a surface. The pigmentation near the tip of the rhizina is due to production of a purple-red pigment by the hyphal cells in this region. |
||||
Physiology of lichens The mycobiont has two principal roles in the lichen symbiosis:
The photobiont also has two roles:
Thus, through the lichen partnership, the photobionts are protected and able to grow in conditions in which they could not grow alone; they also benefit from the highly efficient uptake of mineral nutrients by the lichen fungi. The fungi, in turn, obtain sugars and in some cases organic nitrogen from the photosynthetic partner, enabling them to grow in environments deficient in organic nutrients. |
||||
Water relations of lichens Lichens are remarkable for their ability to withstand prolonged drying and to resume activity rapidly after rewetting. Most lichens that contain green algae can recover from drought by absorbing water from humid air and then begin to photosynthesise. However, the lichens that contain cyanobacteria can only resume photosynthesis after absorbing free (liquid) water. The drought-tolerance of lichens is likely to be conferred by a water-repellent (hydrophobic) coating on the hyphal walls of the medulla. Small peptides that are rich in sulphur-containing amino acids have been found on the hyphal walls of many free-living fungi. They are termed hydrophobins and they probably also occur in lichen fungi. The presence of these compounds would ensure that the medulla around the photosynthetic cells does not become waterlogged, allowing the diffusion of gaseous carbon dioxide for photosynthesis. Of interest, the hydrophobic materials seem to be produced only by the fungus, because cells of Trebouxia, which have a naturally hyrophilic surface, become covered with a hydrophobic material when grown in the presence of a lichen fungus. The rewetting of lichens is thought to start by water absorption in the gelatinous matrix of the cortex, after which water might move through the medulla in the wall space of the fungal hyphae or perhaps by capillarity in regions where the hyphae have a hydrophilic surface. |
||||
Nutrient exchange between the lichen partners Most attention to nutrient exchange in lichens has centred on the mechanisms of carbon flow from the photosynthetic partner to the fungus, because radioactive labelling studies have shown that both green algae and cyanobacteria can release up to 90% of their photosynthate to the fungal partner. In lichens with Trebouxia, and presumably also with other green algae, the fungal hyphae can produce short branches that penetrate through the algal wall to serve as nutrient-absorbing haustoria. This is similar to the behaviour of biotrophic plant pathogens (see Biotrophic Plant Pathogens). In contrast, in lichens with cyanobacteria (e.g. Peltigera) there is no penetration of the photosynthetic cells. Instead, the fungal hyphae of the central region (usually hydrophobic) produce thin-walled protrusions that penetrate the hydrophilic gelatinous sheaths that surround the cyanobacterial cells. The major soluble carbohydrates in lichens are sugar alcohols (polyols). In the fungal partner these compounds are present as mannitol and, to a lesser degree, arabitol. In these respects lichen fungi are no different from other fungi, which characteristically have sugar alcohols as the main soluble carbohydrates. The green algae produce sugar alcohols as their main photosynthetic products - e.g. the sugar alcohol ribitol is produced by Trebouxia. However, the cyanobacteria seem to release glucose to the fungal partner. This seems to occur passively through a glucose carrier in the cell membrane, after enzymic degradation of an intracellular glucan (glucose polymer) in the cyanobacteria. Of interest, the maximum rates of nutrient release from the photosynthetic partner occur in optimal moisture conditions, whereas the photosynthetic cells retain most of their carbohydrate in conditions of water stress. So, it has been suggested that cycles of wetting and drying may be advantageous in maintaining a lichen symbiosis, because both partners could gain sufficient carbohydrate at different stages of this cycle. |
||||
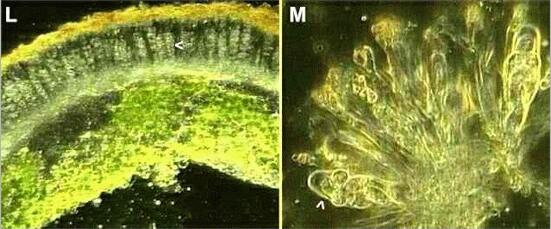 Figures L, M. The fungal sporing stage of Xanthoria parietina. (L) Section through part of a thallus in the region of a disk-shaped apothecium. The apothecium consists of many club-shaped asci (one ascus is marked by an arrowhead) with sterile "packing" hyphae (paraphyses) between the asci. The tips of the paraphyses are orange-pigmented (top of the section). (M) Part of the apothecium crushed to show the asci (one marked by an arrowhead), each containing 8 oval ascospores, and the orange-tipped paraphyses. |
||||
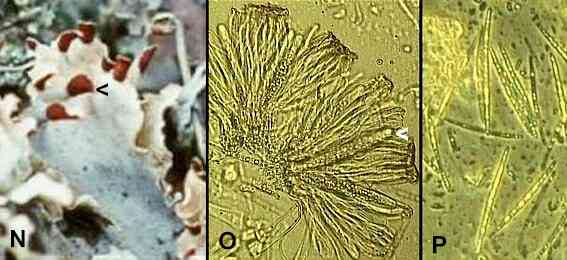 Figures N-P. The fungal sporing stage of Peltigera polydactyla. (N) Apothecia are seen as brown, shield-like structures at the tips of the thallus (this Figure is a close-up of Figure B). (O) Part of a crushed apothecium showing asci (one marked by arrowhead) and brown-tipped paraphyses. (P) Ascospores released by crushing an apothecium. In this lichen the ascospores are needle-shaped, unlike the oval ascospores of Xanthoria. |
||||
Reproduction and dispersal mechanisms Most lichens are dispersed by vegetative propagation. The dry lichen thallus is brittle, so fragments can be broken off easily and transported by wind or by animals. In addition, several lichens produce stalk-like or pillar-shaped structures termed isidia, which are easily broken off and dispersed. All these fragments can resume growth in a new environment after they are rewetted. A further means of propagation is by the production of specialised dispersal units termed soredia (Figures Q, R). These consist of a few photosynthetic cells enveloped in fungal hyphae, and they are formed as a powdery mass near the centre of a lichen thallus or (e.g. Parmelia physodes, Figure A) at the tips of some of the thallus lobes. Soredia are readily dispersed by wind. In addition to these dispersal methods, many lichens produce apothecia or other fungal fruiting structures, to disperse the spores of the fungal partner (Figures L-P). The spores in these cases (ascospores) are formed in asci as the result of sexual reproduction (see The Fungal Web), although a few lichen fungi produce asexual dispersal spores. When these spores germinate they must contact the cells of a photosynthetic partner to establish a new lichen thallus. This process of "reassembly" of a lichen can be demonstrated in experimental conditions, and evidence suggests that it also occurs in nature. But its frequency in natural conditions may vary substantially between different types of lichen. For example, the separate dispersal of fungal and algal (Trebouxia) spores might be the major means of dispersal for the common lichen Xanthoria parietina (see Figure J) and for Buellia species because these lichens do not seem to have soredia. In these cases there is immunological evidence to suggest that lichens are synthesised in nature from free-living Trebouxia and fungal spores on rock surfaces (Mukhtar et al., 1994). |
||||
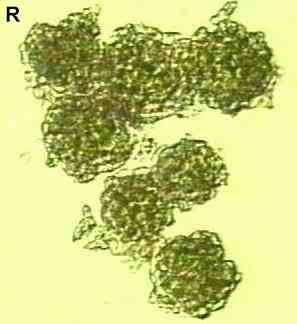
 Figure Q. Physcia grisea, a common lichen that grows on walls, trees and fence posts in city environments. The colony margins consist of narrow lobes (about 2-3 mm) but the centre of each thallus has a darker, greener appearance and is more granular, because it is covered with soredia.
Figure R. Seven soredia seen at high
magnification. Each is about 0.1 mm diameter and consists
of a cluster of algal cells in a meshwork of hyphae. |
||||
Lichen ecology and significance Lichens are amongst the slowest-growing organisms, but their tolerance of environmental extremes enables them to colonise habitats where few other macroscopic organisms can grow. They grow where neither the fungal partner nor the photosynthetic partner could survive alone, because they benefit from their unique symbiotic association. However, most lichens are highly intolerant of atmospheric pollution, particularly sulphur dioxide, so they are found mainly in rural environments rather than cities. Only a few species, such as Physcia grisea and Xanthoria parietina, are found commonly in towns, and even they grow poorly in towns compared with in non-polluted environments. Part of the reason for this intolerance is the extreme efficiency of lichen fungi in accumulating nutrients from trace levels in the atmosphere. An extreme demonstration of this is the ability of lichens to accumulate radioactive isotopes from the environment. Following the Chernobyl disaster, the lichens (mainly Cladonia rangiferina, the "reindeer moss") of northern Scandinavia accumulated so much radioactivity that reindeer feeding on them were considered dangerous for human consumption. |
||||
| Further reading (1) Books, journals and major reference sources Hale ME (1983) The Biology of Lichens. Edward Arnold, London [The best general book on lichen biology] Nash TH (1996) Lichen Biology. Cambridge University Press, Cambridge. [An update of Hale's book, with chapters by different authors, but highly detailed - not recommended for beginners] The main journal for all aspects of lichens is The Lichenologist, published by Academic Press. Many detailed aspects of lichen biology can be found in the three volumes of CRC Handbook of Lichenology (ed. M. Galun), 1988. CRC Press, Boca Raton, Fl, USA. (2) Useful WWW sites Access many lichen sites through the home page of the International Association of Lichenologists (not on this server) See also: Lichen photos (not on this server) And a "lighter" but useful site: Fun with Lichens (not on this server) (3) Selected references for specific topics: Synthesis of lichens in laboratory conditions, and in nature:
Movement of radionuclides from lichens through the animal food chain:
Contribution of lichens to nitrogen-enrichment of various ecosystems:
Lichen physiology:
Lichen interactions and community development:
|
||||