..
CHAPTER 5 : DIFFERENTIATION
AND DEVELOPMENT This chapter is divided into the following major sections:
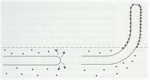
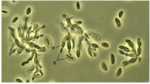
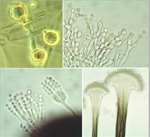
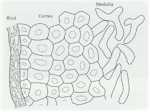
Differentiation can be defined as the regulated change of an organism from one state to another. These states can be physiological, morphological or both. So the germination of fungal spores (Chapter 10) and the switch from primary to secondary metabolism (Chapter 7) are examples of differentiation. But here we focus on the developmental changes that lead to the production of a wide range of differentiated structures, such as the infection structures of fungal pathogens, the regulation of sexual and asexual development, the switch between hyphal and yeast forms of some human-pathogenic fungi, and other developmental processes. We consider both the underlying control mechanisms and the functions of the differentiated structures. MOULD-YEAST DIMORPHISM Most fungi grow either as hyphae (the mycelial, mould, or M-phase) or as single-celled yeasts (the Y-phase). In general, yeasts and yeast phases are found in environments with high levels of soluble sugars that can diffuse towards the cells, or where the cells can be dispersed in liquid films or circulating fluids to obtain nutrients. Yeasts have little or no ability to degrade polymers such as cellulose or proteins, etc. and they also have no penetrating power, unlike the mycelial fungi which commonly have these abilities. So, the yeast form and the mycelial form represent two different growth strategies, suited to particular environments and conditions. However, some fungi can alternate between a mycelial form and a yeast-like form, in response to environmental factors. These dimorphic fungi (with two forms) include several pathogens of humans. For example, Candida albicans commonly grows as a yeast on the mucosal membranes of humans, but converts to hyphae for invasion of host tissues (Chapter 1; see Fig. 1.4). This dimorphic switch can be induced experimentally by growing C. albicans in horse serum of low nutrient content (Chapter 16). Similarly, the fungi such as Metarhizium and Beauveria spp., which commonly parasitise insects, penetrate the insect cuticle by hyphae but then proliferate in a single-celled form in the circulating fluids of the host (Chapter 15). As a further example, the vascular wilt pathogens of plants (e.g. Fusarium oxysporum, Ophiostoma novo-ulmi) penetrate initially by hyphae but then spread as yeast-like forms in the xylem vessels (Chapter 14). The switch between mycelial (M) and yeast-like (Y) growth of dimorphic fungi occurs in response to environmental factors, and can be reproduced experimentally as shown in Table 5.1. Several opportunistic pathogens of humans grow in the M phase as saprotrophs in plant and animal remains (their normal habitat, Chapter 16) and also grow as mycelia in laboratory culture at 20-25oC. But they convert to budding yeasts or swollen cells in the body fluids or when grown at 37oC in laboratory culture. This thermally-regulated dimorphism is a significant factor in human pathogenesis. In contrast, the dimorphic saprotrophic Mucor species (e.g. M. rouxii, M. racemosus) do not respond to temperature changes but respond to oxygen levels. They grow as budding yeasts in anaerobic conditions but as mycelia in the presence of even low concentrations of oxygen. Ustilago maydis and other plant-pathogenic smut fungi (Basidiomycota) are yeast-like in their monokaryotic phase but hyphal in the dikayotic form (see Chapter 2) so their transition is governed genetically. This range of responses shows that there is no common environmental cue that governs the M-Y transition, and instead we need to consider the underlying control mechanisms.In this regard, the studies on cell polarity of budding yeasts and fission yeasts (Chapter 4) are particularly important because several of the genes that regulate polarity have been identified.
Table 5.2. Roles of mating type genes in Basidiomycota
-------------------------------
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||