..
CHAPTER 6: FUNGAL NUTRITION This chapter is divided into the following major sections:
SAMPLE TEXT Fungi have quite simple nutritional requirements. They need a source of organic nutrients to supply their energy and to supply carbon skeletons for cellular synthesis. But, given a simple energy source such as glucose, many fungi can synthesise all their other cellular components from inorganic sources - ammonium or nitrate ions, phosphate ions and trace levels of other minerals such as calcium, potassium, magnesium and iron. Fungi that normally grow in a host environment or in other nutrient-rich substrates might require additional components, but still the nutrient requirements of most fungi are quite simple. Having said this, fungi need to capture nutrients from their surroundings. The cell wall prevents fungi from engulfing food particles, so fungi absorb simple, soluble nutrients through the wall and plasma membrane. In many cases this is achieved by releasing enzymes to degrade complex polymers and then absorbing the nutrients released by these depolymerase enzymes. Fungi produce a huge range of these enzymes, to degrade different types of polymer. In fact, there is hardly any naturally occurring organic compound that cannot be utilised as a nutrient source by one fungus or another. In this chapter we consider the many adaptations that fungi have evolved for nutrient-capture. Table 6.1. A chemically defined liquid culture medium for fungi
----------------------------
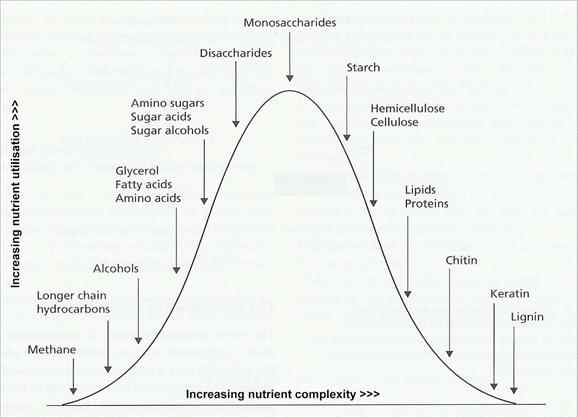
Fig. 6.1. Some of the major carbon substrates of fungi [© Jim Deacon]
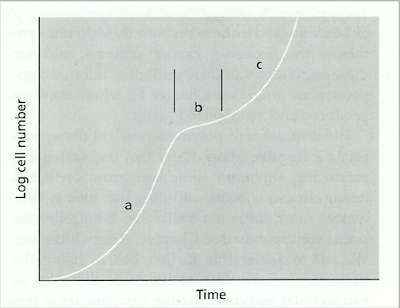
Fig. 6.2. A diauxic growth curve of a fungus grown on the disaccharide lactose: (a) initial phase of growth on glucose, (b) temporary slowing of growth while a galactose transporter is inserted in the membrane; (c) resumed growth on galactose. [© Jim Deacon]
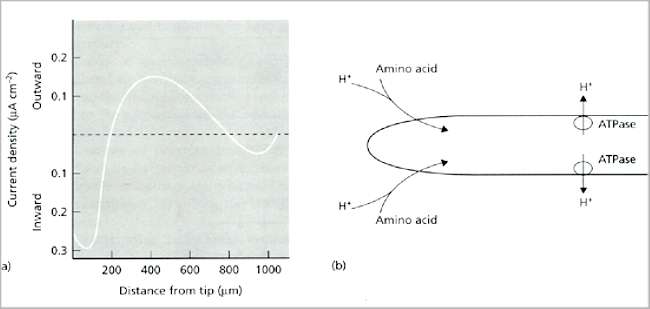
Fig. 6.3. (a) Typical current profile around an individual hypha of a growing fungus (Achlya sp.) [From Gow, 1984]. (b) Interpretation of the current, involving proton export through ATPase-driven proton pumps behind the hyphal tip, and uptake of protons at the hyphal tip through symport proteins that simultaneously transport amino acids. [Based on a diagram in Kropf et al., 1984, Journal of Cell Biology 99, 486-496]
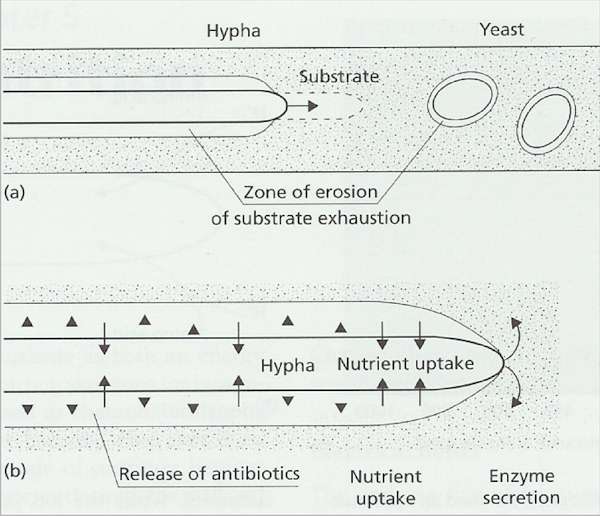
Fig. 6.4. Fungal
strategies for growth on insoluble polymers. (a)
Hyphae extend continuously at the apex, drawing
protoplasm forward to evacuate the zones of enzyme
erosion of the substrate. Yeasts do not utilise insoluble
(non-diffusible) polymers because they would become
trapped in their own substrate erosion zones. (b)
Suggested defence of a substrate by a hypha of a
polymer-degrading fungus. Enzymes are secreted at the
apex to degrade the polymer, and the soluble nutrients
released by these enzymes are absorbed sub-apically.
Antibiotics or other inhibitors (shown as arrowheads) may
be released sub-apically into the substrate erosion zone
to prevent competing organisms from using the enzyme
digestion products.
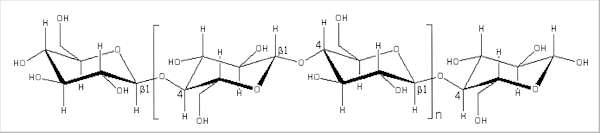
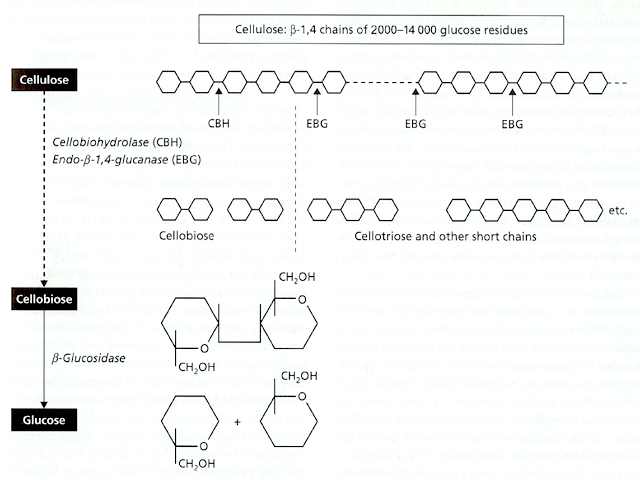
Fig. 6.5. Structure of a single cellulose chain, composed of thousands of repeated disaccharide (cellobiose) units; one disaccharide unit is shown within the brackets. The ß1-4 bonds are also shown.
Fig. 6.6.
Structure and enzymatic breakdown of cellulose. [©
Jim Deacon] At least three types of enzyme are involved in the complete breakdown of a cellulose chain to its constituent glucose units, and some of these enzymes exist in multiple forms of different molecular mass. Collectively they are termed ‘cellulase’ or, more precisely, the cellulase enzyme complex. The three main enzymes in this complex are: (1) An endoglucanase (endo-ß-1,4-glucanase), which acts at random points within a cellulose chain, breaking the molecule into successively smaller fragments. This enzyme is found in multiple forms, ranging from about 11,000 to 65,000 Da. (2) An exo-acting enzyme, termed cellobiohydrolase, which acts only on the ends of cellulose chains, cleaving off successive disaccharide units (cellobiose). This enzyme is more uniform than the endoglucanase, ranging from about 50,000 to 60,000 Da. (3) ß-glucosidase (or ‘cellobiase’), which cleaves the disaccharide cellobiose to glucose, for uptake by the fungus. This is a wall-bound enzyme, like many enzymes involved in the final stages of degradation of polymers. All three enzymes act synergistically and are tightly regulated, to ensure that a cellulose-degrading fungus does not release sugars at a faster rate than it can use them. The endoglucanases, by attacking the cellulose chains at random, progressively create more ends on which cellobiohydrolase can act. But the resulting cellobiose can bind to the active site of cellobiohydrolase, and competitively inhibit the enzyme action. Thus, if cellobiose accumulates then the rate of cellulose breakdown will automatically be slowed, and this regulation links the rate of breakdown to the rate at which the fungus requires glucose for growth and metabolism. In addition to this, the regulation of cellulose breakdown is achieved by the common feedback system termed catabolite repression, whereby genes encoding the enzymes are repressed when more readily utilisable substrates like glucose are available.
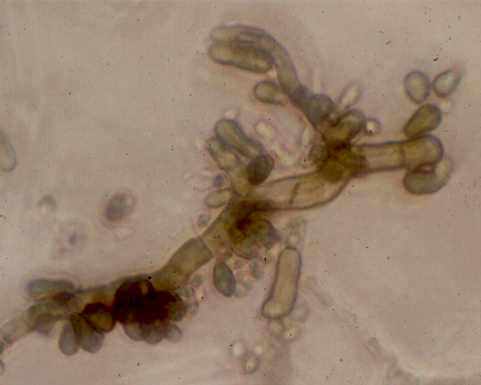
Fig. 6.7. Sydowia polyspora (more commonly known as Aureobasidium pullulans), a darkly-pigmented fungus that commonly grows in poorly ventilated environments such as on kitchen and bathroom walls. The hyphae produce large numbers of pigmented, yeast-like buds. This fungus lacks the enzymes to degrade complex forms of cellulose, but it can grow readily on "soluble celluloses" such as carboxymethyl cellulose, which is one of the main constituents of wallpaper pastes and emulsion paints. [© Jim Deacon]
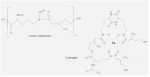
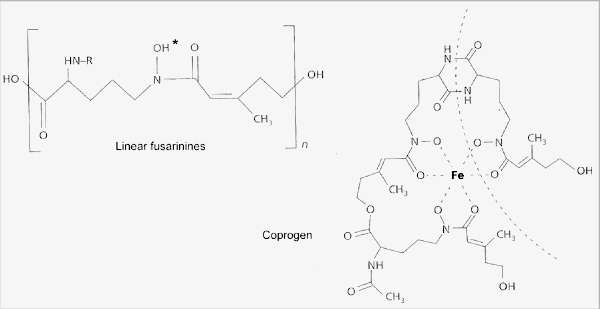
Fig. 6.8. The hydroxamate siderophores of fungi, used to capture ferric iron. The simplest of these are the linear fusarinines, widely found in species of Fusarium, Penicillium and Gliocladium. They sequester iron at the position marked by the asterisk. More complex siderophores such as coprogen have several fusarinine units that bind iron in a ring-like structure (one of these units is shown by the broken line). [© Jim Deacon] |
|||||||||||||||||||||||||||||||||||||||||