Biofilms Although many bacteria can grow in a free-living, 'planktonic' state, it is quite common for them to adhere to surfaces by producing extracellular polysaccharide or in some cases by means of specialised structures termed holdfasts. The adherent bacteria produce microcolonies, leading to the development of biofilms, which initially may be composed of only one bacterial type, but frequently develop to contain several bacteria living in a complex community. In fact, almost every surface exposed to liquids and nutrients will be colonised by microorganisms. A classic example is the biofilm on our teeth, leading to the development of cavities (dental caries) when bacteria such as Streptococcus mutans degrade sugars to organic acids. Biofilms also are found on the solid supports in sewage-treatment plants, where they play an essential role in processing of sewage water before it is discharged into rivers. And biofilms are found on the surfaces of water tanks, pipes, surgical apparatus, food-processing vessels, etc., where the bacteria adhere tenaciously, resisting removal by washing and also gaining protection from the common disinfectants which cannot easily penetrate the polysaccharide matrix. |
|||||
 Images supplied by: DG Allison & IW Sutherland |
|||||
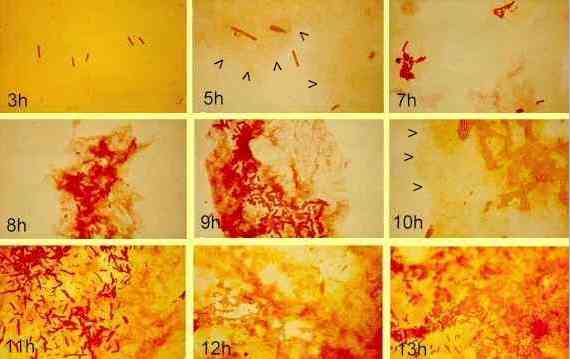
In the sequence above, a Gram-negative bacterium (Pseudomonas strain S61) was allowed to develop as a biofilm on glass slides immersed in nutrient medium containing 1% glucose. Development of the biofilm was followed by a specific staining technique (Congo red) in which the bacterial cells stain dark red and the exopolysaccharide stains orange-pink (DG Allison & IW Sutherland, 1984, Journal of Microbiological Methods 2, 93-99). Attached cells can be seen at 3 hours. They then divide and form microcolonies. After 5 hours the development of exopolysaccharide is clearly seen (arrowheads) and it increases as the microcolonies increase in size. |
|||||
 Image supplied by: Lucy Skillman |
|||||
Techniques such as this can be used to ask many interesting and important questions such as:
|
|||||
An interesting type of biofilm is produced by some of the highly specialised methane-oxidising bacteria (methanotrophs). These bacteria can be cultured in flasks containing a simple mineral nutrient medium, with a mixture of methane gas (CH4) as the sole carbon source and oxygen in the headspace (left-hand image above). The bacteria produce a pink-coloured, tough film resembling a sheet of polyethylene at the surface of the liquid (it has been disturbed and has become folded in the flasks shown here). The bacterial cells grow as a monolayer within this film (right-hand image), with block-like patterns of cells representing their planes of division. Vast amounts of methane are produced in nature (in the rumen of cows, landfill sites, etc.) by anaerobic archaea (the methanogens) which gain energy by oxidation of hydrogen coupled with the reduction of carbon dioxide, to produce methane. The accumulation of methane trapped in geological deposits has produced the vast natural gas fields that are exploited as a fuel source today. In the absence of entrapment, methane diffuses into aerated sites in soil, where it is metabolised by the methylotrophs (mostly Gram negative cocci or plump rods of genera such as Methylococcus and Methylomonas). By forming a surface film at the interface between anaerobic and aerobic zones, these bacteria have a supply of methane gas (poorly soluble in water) and also a supply of oxygen essential for utilising methane. Most of these bacteria cannot use more complex organic compounds containing C-C bonds. |
|||||
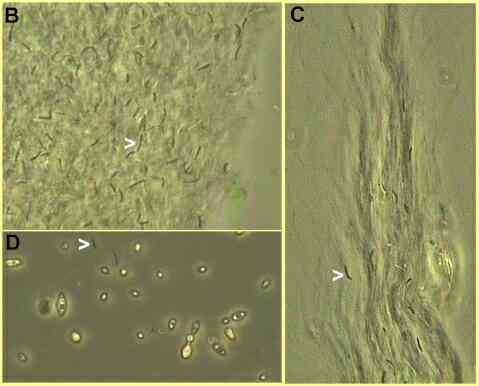
Kombucha tea Some members of the bacterial genus Acetobacter, especially Acetobacter xylinum, synthesize large amounts of cellulose when grown on sugar sources. These bacteria occur naturally on the surfaces of fruits and flowers, and have important roles in the commercial production of vinegar from wines and other fermented products. They are strictly aerobic (oxygen-requiring) organisms and they often grow after a phase of activity by fermentative organisms (e.g. yeasts), converting the fermentation end products to more oxidised forms. For example, one of their characteristic activities is to oxidise ethanol to acetic acid, so they are commonly known as acetic acid bacteria. One of the more unusual roles of these bacteria is in the production of fermented teas, such as Kombucha tea. This tradition dates back more than 2000 years in eastern countries such as China, Japan and Russia. Recently it has become popular as a "herbal remedy" in western societies. (see The Kombucha Network (not on this server) The tea is made by adding a microbial culture to a mixture of black tea (cooled) and sugar, with a small amount of vinegar to acidify it. Then the mixture is incubated for 1-2 weeks. During this time, Acetobacter and various yeasts grow to produce a rubbery, pancake-like mass on the surface of the tea (Figure A below).
Figure A. Bowl of Kombucha tea after 2 weeks' incubation (left) and the rubbery mat removed from this culture (right) This pancake has various popular names such as "tea fungus" or "Magical mushroom" but in fact is a meshwork of cellulose fibres with embedded bacterial cells and some yeasts (Figures B-D below). A portion of it is used as inoculum to produce the next batch of tea. A wide range of metabolic products of the acetic acid bacteria accumulate in the liquid medium, including acetic acid, ketones and gluconic acids. The types and proportions of these compounds will vary with time and culture conditions. Perhaps this variation accounts for the astonishingly wide range of effects that Kombucha is claimed to have - from relief of athlete's foot or removal of warts to the elimination of ageing, restoration of hair colour and cure of cancers!
Phase-contrast micrographs of part of a Kombucha mat. B and C show separately spaced cells of Acetobacter xylinum (arrowheads) embedded in extensive sheaths of cellulose microfibrils that they synthesized. D shows a few bacterial cells (e.g. arrowhead) and several budding yeast cells in the free liquid below the mat. Warning: The almost cult status of Kombucha tea has led to networks for the sharing of "starter cultures" which might contain potentially harmful contaminants. To some degree, the activities of the mixed population of organisms in a Kombucha culture serve to regulate the culture environment (e.g. lowering of the pH) and to reduce the growth of contaminants. But the typical domestic kitchen is not a microbiologist's model of a clean and safe environment! It is unlikely that either microbiologists or qualified medical practitioners would endorse the drinking of Kombucha tea. No endorsement is implied in this site. |
|||||
Further reading: Books
Research articles
Websites
|
|||||