..
CHAPTER 4 IMAGES: FUNGAL GROWTH
Figure 4.1 illustrates part of this plasticity, when hyphae of Neurospora crassa are observed by placing a coverslip over the margin of a colony on an agar plate. The sequence of 9 frames was taken over a one-hour period, starting from the time when the coverslip was added. In the first frame (a) the hyphal tip was growing normally, and two lateral branches had arisen behind the growing tip - the normal behaviour of a fungal hypha. Soon afterwards (b and c) the hyphal tips began to swell (a response to disturbance caused by the coverslip) and then branched repeatedly from the tips. There are 3 points to note: (1) The disturbance caused by adding the coverslip caused an immediate change in behaviour - the hyphae started to branch at their tips, not behind their tips. (2) The hyphae originally had a smooth profile, but adding the coverslip caused the hyphae to grow irregularly (perhaps because of the lower oxygen availability). (3) Fungal hyphae grow only at their extreme tips, and the hyphal wall rigidifies quite rapidy behind the tip. We can see this by comparing frames d and i - the distance between points 1 and 2 remained constant.[© Jim Deacon]
Fig. 4.2. Incorporation of radiolabelled wall precursors during a brief (5 minute) exposure. [© Jim Deacon]
Fig. 4.3. Noel Robertson’s experiments on hyphal tip growth. See text for details. [© Jim Deacon]
Fig. 4.4. Diagrammatic representation of the possible organisation of wall growth at the hyphal apex. Only half of the hypha is shown. Vesicles (V) derived from the endoplasmic reticulum and Golgi body (G) are transported to the apex, probably by microtubule (M) -associated motor proteins. The vesicles could then be directed to the plasma membrane, perhaps by actin-associated motor proteins. The newly-formed wall at the extreme hyphal tip is thin and has few cross-linkages, but becomes increasingly cross-linked further back. In contrast, the actin cytoskeleton is highly delevoped at the extreme tip (see Chapter 3; Fig. 3.19) and might help to provide structural support, compensating for the lack of wall cross-linking at the tip. The concentration of actin progressively decreases behind the tip. [© Jim Deacon]
Fig. 4.5 illustrates some of the components of wall synthesis at the hyphal tip. Vesicles are thought to deliver the main wall-synthetic enzymes (chitin synthase and glucan synthase) to the tip, where they lodge in the plasma membrane as integral membrane proteins. Mannoproteins and other glycoproteins are transported in vesicles from the Endoplasmic reticulum - Golgi secretory system (because the glycosylation of proteins occurs only in the Golgi). Multivesicular bodies, whose functions are still unclear, may be carried as vesicular cargoes along microtubules. Enzyme activators and inhibitors also are thought to be involved in the orchestration of tip growth, but the substrates for wall synthesis arrive from metabolic reactions in the cytosol. [© Jim Deacon]
Fig. 4.6. Representation of the steady state model of hyphal tip growth, in which the wall is envisaged as being visco-elastic. New wall polymers synthesised at the extreme tip are suggested to flow outwards and backwards as new components are continually added at the tip. The decreasing thickness of the arrows behind the tip signifies progressively reduced flow as the polymers become cross-linked. [Based on a diagram in Wessels, 1990.] [© Jim Deacon]
Fig. 4.7. Stages in germination of spores of Aspergillus niger. (a) In normal conditions (e.g. 30oC) the spore swells and incorporates new wall material over the whole of the cell surface (shown by stippling), then a germ-tube emerges and all new wall incorporation is localised to the hyphal tip. (b) At 44oC the spore continues to swell and incorporates wall material in a non-polar manner, producing a giant cell with a thick wall. If the temperature is lowered to 30oC this cell produces an outgrowth, which immediately differentiates to produce a spore-bearing head. [Based on Anderson & Smith, 1971.]
Fig. 4.8. Germination behaviour of spores of Geotrichum candidum, when incubated in a thin water film beneath a cover-slip. The spores always germinate from positions near their poles. Arrows indicate the positions of germ-tube outgrowth in different conditions. (a – d) Negative autotropism of spores touching in pairs or in groups – the spores always germinate from a position furthest from a touching spore. (e) The presence of oxygen (a small hole in the coverslip) negates the negative autotropism – the spores germinate from a point closest to the oxygen source. [Redrawn from Robinson, 1973.]
Fig. 4.9. Behaviour of spores (S) of Idriella bolleyi on wheat root hairs in aseptic conditions. A, spores germinate towards a dead root hair, and then envelop and penetrate the root hair. B, spores germinate away from a living root hair (rh). From Allan et al., 1992. [See further images below] [© Jim Deacon]
Fig. 4.9a. Spores (S) of Idriella bolleyi that germinate towards living wheat root hairs in aseptic conditions can produce further spores within 24 hours. Such spores can rapidly spread down the soil profile in field conditions. From Allan et al., 1992. [© Jim Deacon]
Fig. 4.9b. Demonstration of the different modes of behaviour of spores of Idriella bolleyi. Spores lying next to the living root hair (which contains protoplasm - seen towards the left of the image) have germinated away from the root hair, whereas spores lying next to the dead root hair in the same field of view are penetrating the root hair. [© Jim Deacon]
Fig. 4.10. Left: Orientation of hyphal tips and orientated emergence of hyphal branches of Achlya and Saprolegnia spp. towards an agar disk (black circle) containing a mixture of amino acids. Right: Reorientation of hyphae from germinating spores of Pythium aphanidermatum towards a mixture of amino acids (Mitchell & Deacon, 1986). [© Jim Deacon]
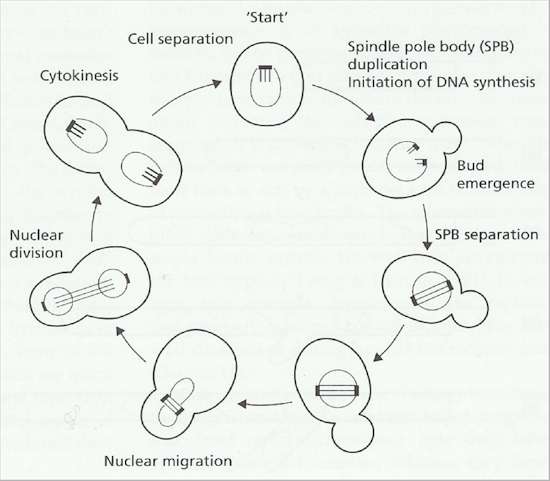
Fig. 4.11. Events in the cell cycle of Saccharomyces cerevisiae. [based on a drawing by Hartwell, 1974]
Fig. 4.13. The duplication cycle of Basidiobolus ranarum, a fungus that grows as hyphae with complete, unperforated septa on agar plates. a-b: an apical cell extends, synthesises new protoplasm and continually draws the protoplasm forwards. c: when the protoplasmic volume attains a critical size the nucleus divides and a septum is formed. d-e: The new apical cell grows on and repeats the process; the sub-apical cell produces a branch, and the protoplasm and nucleus migrate into this, producing a second apical cell. [© Jim Deacon]
Fig. 4.15. Typical growth curve of a batch culture. (a) Lag phase; (b) exponential or logarithmic growth phase; (c) deceleration phase; (d) stationary phase; (e) phase of autolysis. [© Jim Deacon]
Fig. 4.16. Representation of a continuous culture system to produce fungal biomass [© Jim Deacon]
|
||||||||||||||