..
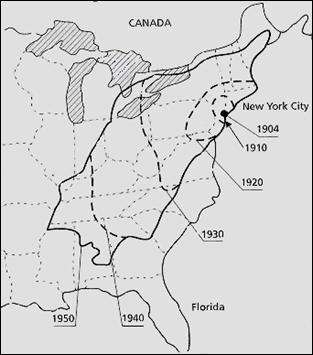
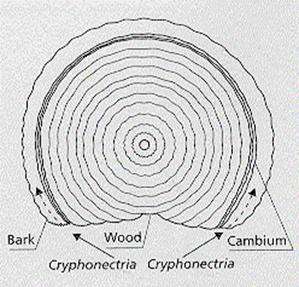
MAJOR TREE PATHOGENS: CHESTNUT BLIGHT (CRYPHONECTRIA PARASITICA) Chestnut blight, caused by the fungus Cryphonectria parasitica (Ascomycota), has destroyed many millions of trees in North America, following the first report of its occurrence in the New York Zoological Garden in 1904 (Fig. 1). Its progressive spread across the eastern half of the USA reduced the native American chestnut (Castanea dentata) almost to the status of an understorey shrub, whereas it was once a magnificent ‘high forest’ tree. However, recent studies raise the prospect that chestnut blight might be controlled by genetic manipulation of hypovirus-associated double-stranded RNA, discussed below. Cryphonectria infects through wounds in the bark of chestnut trees, and then spreads in the cambium, progressively girdling the stem and killing the plant above the infection point (Fig. 2). This disease was also a significant problem in European sweet chestnut trees (Castanea sativa) in the 1940s, but in Italy in the early 1950s some heavily infested sweet chestnut plantations began to recover spontaneously. The lesions in these trees stopped spreading round the trunks, and strains of C. parasitica isolated from them showed abnormal features. They grew slowly and erratically on agar, were white rather than the normal orange colour, produced significantly fewer conidia, and showed only low virulence when wound-inoculated into trees. This low virulence (hypovirulence) could be transmitted to other strains during hyphal anastomosis on agar (i.e. when hyphal tips pf different strains fuse with one another) so it was coined transmissible hypovirulence. None of the hypovirulent strains contained ‘conventional’ virus-like particles, but all the hypovirulent strains contained dsRNA whereas virulent strains had no dsRNA. Electron micrographs show that this dsRNA is contained in rounded or club-shaped, membrane-bound vesicles in the cytoplasm (Newhouse et al., 1983) and that, unlike many virus-like particles, these can occur in significant amounts in the hyphal tips of C. parasitica.
Fig. 1. Recorded spread of chestnut blight caused by Cryphonectria parasitica after it was first recorded in New York in 1904.
Fig. 2. Diagram of a cross-section of a trunk with a spreading canker caused by Cryphonectria parasitica. This fungus produces airborne spores that infect through wounds, then progressively girdles the trunk by growing in the cambium. The spontaneous decline of chestnut blight in Italy led French workers to develop a highly successful biological control programme. Hypovirulent strains of C. parasitica were cultured in the laboratory and inoculated at the expanding margins of cankers in the field. Within a short time the cankers stopped growing, and only hypovirulent strains could be recovered where once the virulent strain had been. This transmissible change of phenotype was always accompanied by the transmission of dsRNA. Following the success of this programme in Europe, attempts were made to introduce hypovirulent strains of Cryphonectria into the USA. But they gave only partial and localised disease control, because the pathogen population in the USA consists of numerous vegetative compatibility groups (VCGs) that limit the natural transfer of dsRNA, owing to cytoplasmic death when the strains fuse with one another. In one early study in the USA, about 0.5 hectare of natural chestnut forest was found to contain at least 35 VCGs. More recently the VCGs have been shown to be in a continuous state of flux: samplings of identical trees over several years showed that some of the predominant VCGs declined, while new ones arose (Anagnostakis, 1992). The European population of C. parasitica is much more uniform in terms of VCGs, and this probably accounts for the success of the biocontrol programme in Europe. Progress in understanding the role of dsRNA in Cryphonectria was significantly delayed by the lack of a suitable transformation system for this fungus. However, when a system was eventually developed it led to rapid progress (Nuss, 1992). The dsRNA of C. parasitica was found to be highly variable, with lengths falling into three broad size ranges - S (small), M (medium) and large (L, about 12.7 kb). As shown in Fig. 3, all these types have the same terminal regions - a poly-A tail at the 3' end and a 28-nucleotide conserved sequence at the 5' end. They differ mainly in the degree of internal deletion. The L-form seems to be the full-length molecule, and the smaller forms are defective (presumably functionless) derivatives which accumulate in the hyphae. This high degree of variability of RNA genomes is not unusual because there is no effective proof-reading system for RNA, to ensure that it is copied faithfully.
Fig. 3. Double-stranded RNA of hypovirulent strains of C. parasitica. Large (L) dsRNA is the full-length molecule comprising two conserved end regions and a central coding region of two open reading frames (ORF A and ORF B) which confer hypovirulence. Medium (M) and small (S) dsRNAs are also commonly found in hyphae. They are internally deleted copies of the L-dsRNA. With the development of a transformation system, Nuss and his colleagues were able to produce a cDNA from the full-length dsRNA and transform it into virulent strains. It caused the virulent strains to become hypovirulent and to exhibit all the features typical of hypovirulent strains - slow growth, pale coloration and reduced sporulation (the hypovirulence-associated traits). Thus, dsRNA was shown unequivocally to be the cause of hypovirulence. The cDNA could also be used for molecular analysis of the dsRNA, which was shown to consist of two open reading frames (ORFs) - ORF A of 622 codons (nucleotide triplets) and ORF B of 3165 codons. When ORF B was deleted from the cDNA and the remaining cDNA was transformed into C. parasitica the fungus was fully virulent. This indicates that ORF A codes for the hypovirulence (disease control) phenotype. In similar tests, deletion of ORF A led to loss of the hypovirulence-associated traits (slow growth, low sporulation, etc.) but not to a loss of hypovirulence. So, it seems that ORF B is necessary for the expression of hypovirulence, whereas ORF A encodes many of the hypovirulence-associated traits which are potentially disadvantageous in a biocontrol strain, reducing its environmental fitness. It may be possible, therefore, to manipulate the cDNA in vitro so that it has only the most desirable traits for biocontrol. It is notable that the cDNA, when transformed into Cryphonectria (causing the fungus to be hypovirulent), became stably integrated in the chromosomal genome so that it was replicated along with the other chromosomal genes. This cDNA was maintained throughout the life cycle, even entering the sexual spores, whereas dsRNA seldom enters the sexual spores of Cryphonectria or other Ascomycota. This would mean that biocontrol strains could be produced with permanent, stable hypovirulence, subject to proof reading like the rest of the chromosomal genes. Recent developments The question arising from the stable integration of ‘hypovirulence’ cDNA is: can there still be cytoplasmically transmitted hypovirulence? The answer seems to be ‘yes’ because the chromosomally integrated cDNA is transcribed into dsRNA rather than into single-stranded mRNA (as in the rest of the genome) and this dsRNA accumulates in the cytoplasm, where it can be transmitted between strains during hyphal anastomosis. This still leaves unanswered the question of how dsRNA suppresses virulence. Preliminary evidence suggests that it does so by down-regulating some of the normal chromosomal genes, including the gene for production of laccase, an enzyme involved in lignin breakdown, which is likely to be significant for a fungus that colonises wood. When the hypovirulence cDNA (derived from dsRNA of C. parasitica) was transformed into other canker-forming Cryphonectria species, and into a less closely related Endothia species, it failed to convert these to hypovirulence. However, when the same cDNA was used as a template to produce RNA and this was transfected into the other fungi, it gave rise to full-length dsRNA in the cytoplasm and caused both a marked reduction of virulence and an alteration of the growth rate and pigmentation (Chen et al., 1994). The success achieved with RNA but not cDNA seems to be explained by the fact that C. parasitica produces RNA from the cDNA but then splices this RNA to delete a 73-base sequence before the RNA can act as a template for dsRNA production. In summary, this work on the hypovirus system of Cryphonectria has raised many issues of fundamental interest in fungal genetics as well as holding the prospect of developing new approaches to plant disease control. However, recent studies in Switzerland (Hoegger et al., 2003) suggest the need for a cautious approach, because the only way of transmitting the hypovirus is by anastomosis, and both the nuclear DNA and the mitochondrial DNA of the donor (biocontrol) strain are introduced into the environment during this process. The mitochondrial DNA of the donor strain was found to be transmitted in nearly one-half of the treated cankers. The nuclear DNA also persisted in the treated cankers but it did not spread beyond them. The major issue that remains to be resolved is the long-term safety of introducing genetically modified strains of pathogenic fungi into the environment, especially if the nuclear DNA is transferred and increases the genetic diversity of the pathogen. |