..
CHAPTER 12: FUNGAL INTERACTIONS - MECHANISMS AND PRACTICAL EXPLOITATION This chapter is divided into the following major sections:
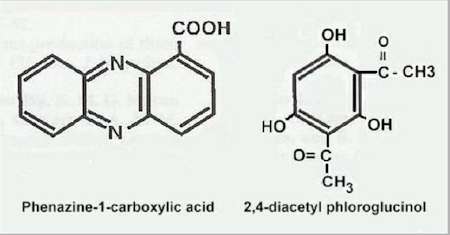
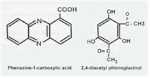
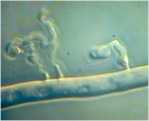
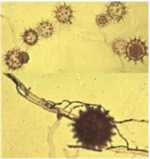
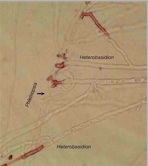
Microorganisms interact in many diverse ways. In this chapter we consider some of the major types of interaction involving fungi, and the potential for exploiting these interactions for practical benefit. We also discuss the use of several commercially available biological control agents. Antibiotics in natural environments: the control of fungi by fluorescent pseudomonads The past two decades have brought major advances in the detection of antibiotics in the root zone (rhizosphere) of crops, helping to explain how fluorescent pseudomonads can control fungal pathogens of roots. Fluorescent pseudomonads are found on the roots of many plants, often at high population levels, and can be detected easily by plating soil dilutions onto “King’s B agar”. This is an iron-deficient medium, so it induces these bacteria to release fluorescent siderophores (iron-chelating compounds) to capture iron (Chapter 6). However, only a small subset of fluorescent pseudomonads (including strains of Pseudomonas fluorescens and P. aureofaciens) are highly effective in controlling fungi. These strains produce specific antifungal antibiotics such as phenazine-1-carboxylic acid (PCA) and 2,4-diacylphloroglucinol (DAPG).
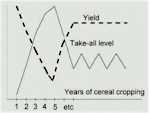
There is clear evidence for a role of antibiotic-producing fluorescent pseudomonads in take-all suppressive soils. An interesting feature revealed by these studies is that the population of antibiotic-producing pseudomonads builds up progressively on wheat crops, but only (or largely) when the take-all fungus is present. Wheat can be grown repeatedly in the absence of the take-all fungus in glasshouse conditions, and this does not lead to a build-up of the antagonistic pseudomonads. So it seems that the crop has to go through a build-up of disease before the disease-suppression sets in.
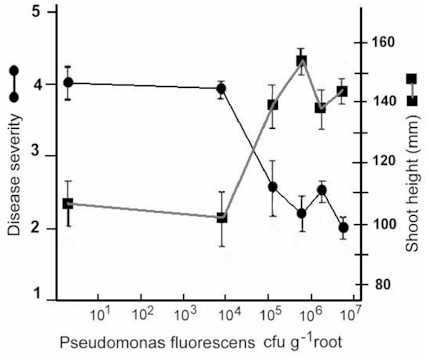
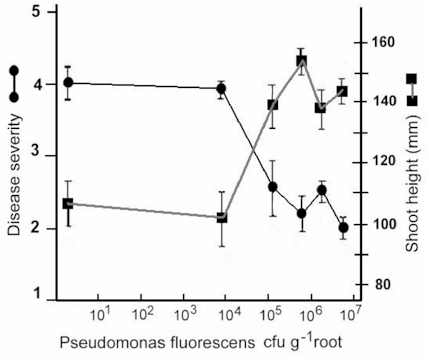
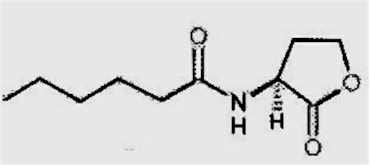
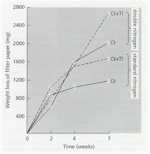
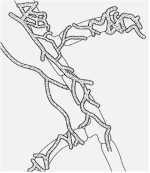
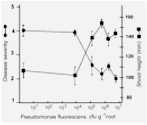
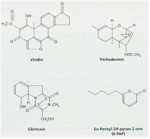
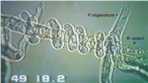
The likely reason for this is shown by the experimental results in Fig. 12.3. Wheat seeds were inoculated with different inoculum densities of DAPG-producing pseudomonads and then sown in a glasshouse in pots of natural soil containing take-all inoculum. The seedlings were sampled after 4 weeks and assessed for disease severity and plant height. There was no disease control until the population level of DAPG strains on the roots exceeded 104 colony-forming units per gram of root. But, at colony levels of 105 and above, there was a marked and significant decrease in disease severity and a corresponding increase in plant height. This “all or nothing” effect is characteristic of a bacterial signalling system called quorum sensing – a term derived from the meetings of committees, where a certain number of people (a quorum) has to be present before a decision can be taken. Quorum-sensing by a population of Gram-negative bacteria involves the continued release of molecules called N-acyl homoserine lactones (Fig. 12.4). When the concentration of these molecules reaches a certain level (indicating that the population is large enough) the relevant genes are switched on. In this case, it is the genes controlling antibiotic production and therefore the control of take-all disease. The production of phenazine antibiotics is known to be under the control of a quorum-sensing system (Chin-A-Woeng et al., 2003), but there is no evidence as yet that DAPG production is regulated in a similar way.
Fig. 12.3. The effects of different population levels of a diacetyl-phloroglucinol producing pseudomonad on disease level (or shoot height) of wheat seedlings grown in take-all infested soil. Data from Raaijmakers & Weller.
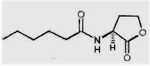
Fig. 12.4. Structure of N-hexanoyl-L-homoserine lactone, a quorum-sensing molecule that regulates the synthesis of phenazine antibiotics by fluorescent pseudomonads.
|
||||||||||||||||||||||||